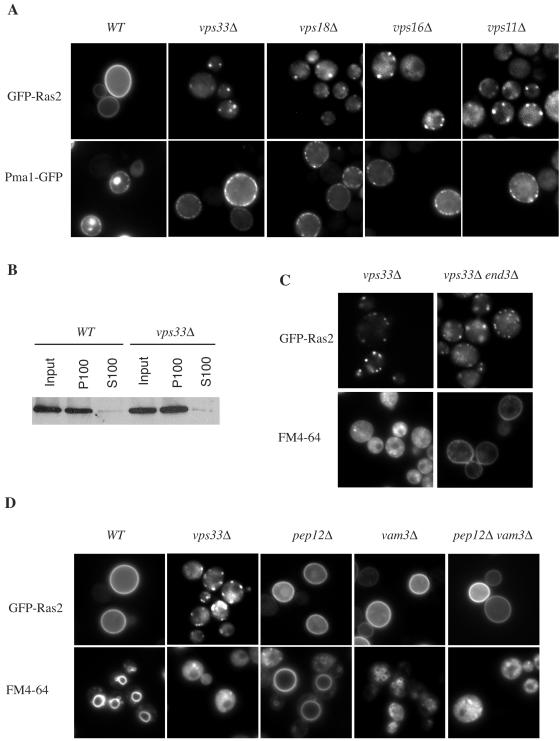
FIG. 3.
Mislocalization of GFP-Ras2 in class C vps mutants. (A) GFP-Ras2 and Pmal-GFP were expressed in wild-type (LRB938) and vps33Δ (RJY1654), vps18Δ (RJY1672), vps16Δ (RJY1695), and vps11Δ (RJY1702) mutant cells by galactose induction and observed by fluorescence microscopy. (B) The membrane association of GFP-Ras2 in wild-type (LBR938) or vps33Δ mutant (RJY1654) cells was assessed by subcellular fractionation of total lysates (input) into cytosolic (S100) and crude membrane (P100) fractions, as described in Materials and Methods. GFP-Ras2 was detected by immunoblotting with an anti-GFP antibody, followed by chemiluminescence (Pierce). (C) Endocytosis does not play a role in endomembrane localization of GFP-Ras in vps33Δ mutant cells. GFP-Ras2 was expressed from a galactose-inducible promoter in the vps33Δ (RJY1654) or vps33Δ end3Δ (RJY1695) mutant strain. Endocytosis was monitored by staining the cells with FM4-64, a dye that is taken up in an End3-dependent manner. (D) Mutations in the class C VPS genes, but not in PEP12 or VAM3, affect the subcellular localization of Ras. GFP-Ras2 was examined in the wild-type (LBR938) and vps33Δ (RJY1654), pep12Δ (RJY1705), vam3Δ (RJY1706), and pep12Δ vam3Δ (RJY1707) mutant strains. FM4-64 was used to monitor endocytosis and to visualize vacuoles.