FIG. 1.
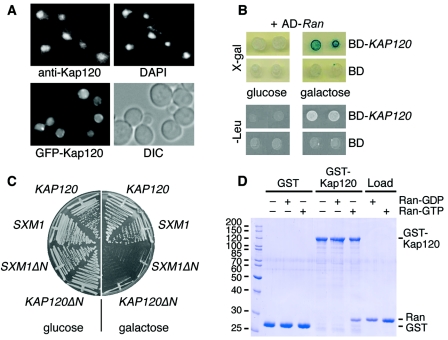
Kap120 is a mostly nuclear protein that interacts with Ran. (A) Kap120 is located predominantly in the nucleus. Wild-type yeast cells were cultured in liquid media at 30°C. The cells were prepared for immunofluorescence microscopy, probed with affinity-purified Kap120-specific antibodies and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies to visualize Kap120, and treated with DAPI to stain the DNA (upper panel). Wild-type cells were transformed with plasmid pGS1233 encoding GFP-Kap120. The synthesis of the fusion protein was induced by addition of 2% galactose to liquid cultures. After 2 h, the cells were viewed by fluorescence microscopy to localize GFP-Kap120 in living cells and also viewed by Nomarski optics/differential interference contrast (DIC) (lower panel). (B) KAP120 interacts with GSP1 by two-hybrid analysis. The yeast strain EGY48 carrying the lexA operon-LEU2 reporter and the lexA operon-lacZ reporter was transformed with plasmid pJG-GSP1 containing a hybrid of the AD and the GSP1 open reading frame under control of a galactose-inducible promoter. This strain was transformed with plasmid pEG-KAP120 encoding a fusion of the lexA DNA BD with full-length Kap120 or with plasmid pEG202 encoding the BD only. Cells of two transformants were spotted onto X-Gal plates or Leu− plates containing either glucose (repression) or galactose (induction) and incubated for 3 days at 30°C. (C) Expression of KAP120ΔN causes a dominant-lethal phenotype. Wild-type cells containing plasmid YCpGAL-KAP120, YCpGAL-SXM1, YCpGAL-KAP120ΔN, or YCpGAL-SXM1ΔN as indicated were streaked on plates containing 2% glucose or 2% galactose and incubated for 2 days at 30°C. (D) Kap120 binds to Gsp1-GTP but not to Gsp1-GDP. Recombinant GST (8 μg per reaction) or a GST-Kap120 fusion protein (14 μg per reaction) purified from E. coli lysates was immobilized on glutathione Sepharose and incubated for 60 min at 4°C with buffer alone, 15 μg of Gsp1-GDP, or 15 μg of Gsp1-GTP as indicated. The bound material of the reactions was washed three times with binding buffer and aliquots were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining together with 20% of the loads of Gsp1-GDP and Gsp1-GTP. Molecular mass markers are given in kDa.