FIG.6.
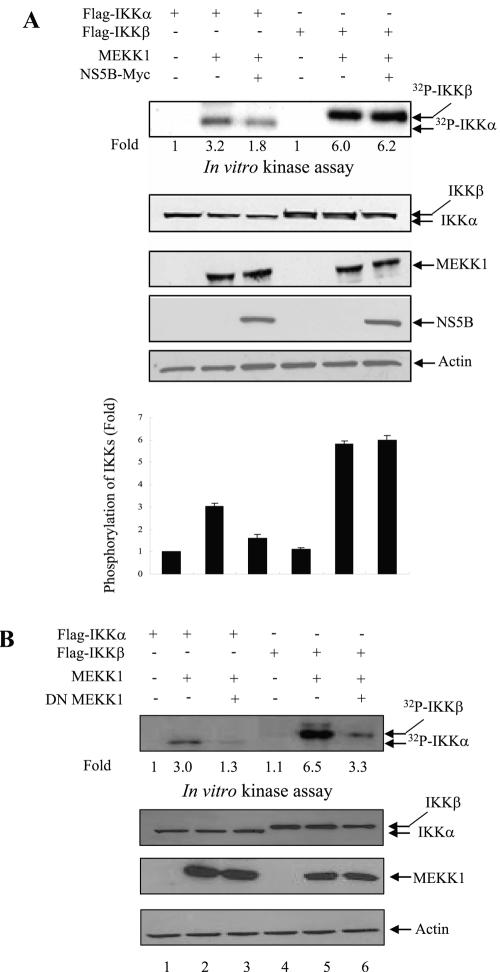
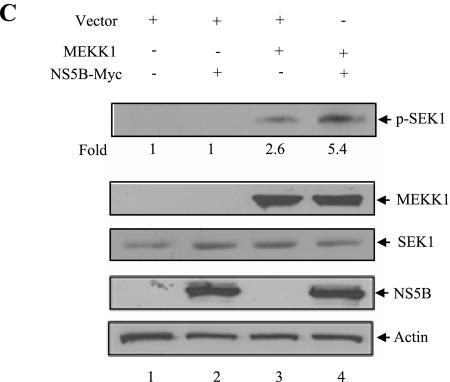
NS5B protein inhibits MEKK1-mediated IKK activity but promotes SEK1 activity. (A) MEKK1-mediated IKK activity was inhibited by NS5B protein. Huh7 cells were cotransfected with either Flag-IKKα or Flag-IKKβ and MEKK1 in the absence (−) or presence (+) of NS5B as indicated. At 36 h after transfection, cell lysates were immunoprecipitated with Flag antibody and proteins coprecipitated with Flag-IKK were assayed for kinase activity using [γ-32P]ATP. The reaction mixtures were resolved by 10% SDS-PAGE and then detected by autoradiography (top panel). Protein expressions of IKKα, IKKβ, MEKK1, NS5B, and β-actin in the same cell lysates were verified by immunoblot analysis (middle four panels). The average IKK phosphorylation is graphically demonstrated from triplicate experimental data (bottom graph). (B) MEKK1 specifically activates both IKKα and IKKβ. Huh7 cells were cotransfected with either Flag-IKKα or Flag-IKKβ, and MEKK1 in the absence (−) or presence (+) of DN MEKK1, and a kinase assay was performed as described for panel A (top panel). Protein expressions of IKKα, IKKβ, MEKK1, and β-actin in the same cell lysates were verified by immunoblot analysis (lower three panels). Since DN MEKK1 has the same molecular mass as active MEKK1 (13), protein expression of DN MEKK1 was examined separately and not shown here. (C) MEKK1-mediated SEK1 activity was synergistically elevated by NS5B protein. Huh7 cells were cotransfected with MEKK1 and HCV NS5B expression plasmids. At 36 h after transfection, cell lysates were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane, and SEK1 activity was determined by immunoblotting with rabbit anti-phospho-SEK1 antibody (top panel). Protein levels in the same cell lysates were determined by immunoblot analysis using antibodies against MEKK1, SEK1, NS5B, and β-actin, individually (lower four panels). The results shown are representative of three independent experiments.