FIG. 1.
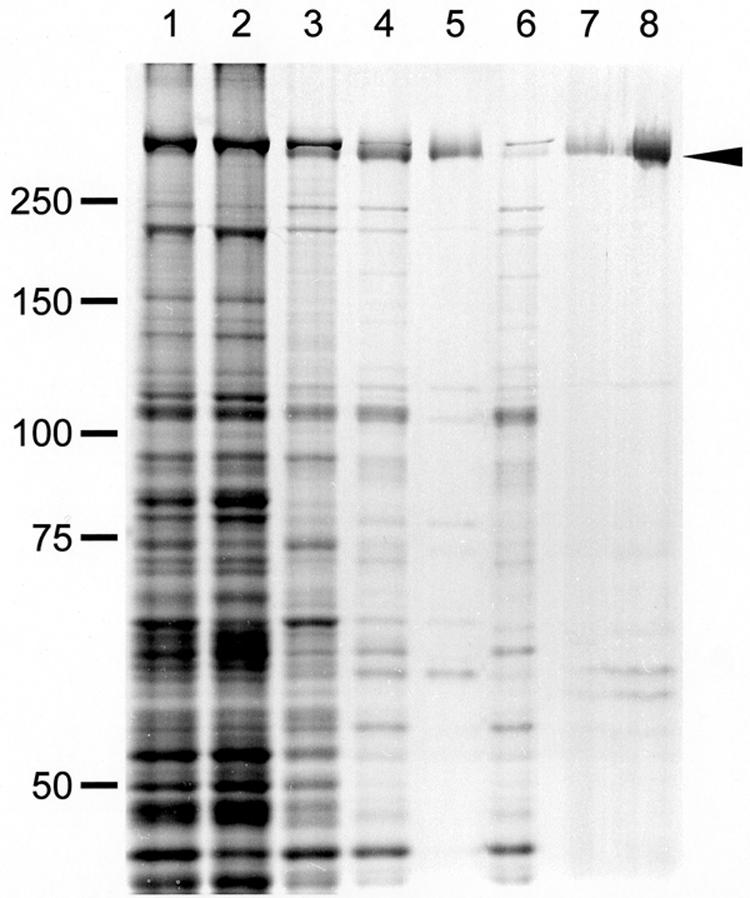
Protein profiles of fractions in the Gli349 purification procedure. Gli349 protein was purified through four steps: (i) Triton X-100 treatment of cells and centrifugation, (ii) stepwise ammonium sulfate fractionation, (iii) precipitation of other proteins by a pH shift from 8.0 to 5.9, and (iv) Q-Sepharose (anion exchanger) column chromatography. The arrowhead shows the Gli349 protein band. Lane 1: whole-cell lysate. Lane 2: Triton-insoluble fraction of step 1. Lane 3: Triton-soluble fraction of step 1. Lane 4: precipitate of 35% saturation ammonium sulfate in step 2. Lane 5: supernatant of step 3. Lane 6: precipitate of step 3. Lane 7: fraction eluted at 0.15 M NaCl in step 4. Lane 8: 10-fold concentration of the fraction in lane 7. Each fraction was subjected to SDS-7.5% PAGE with a 3-mm lane width and stained by the reverse-staining method. Protein fractions derived from 0.2-, 0.5-, and 5-ml cultures were applied to lanes 1 to 6, 7, and 8, respectively. Molecular masses are indicated on the left in kilodaltons.
