FIG. 1.
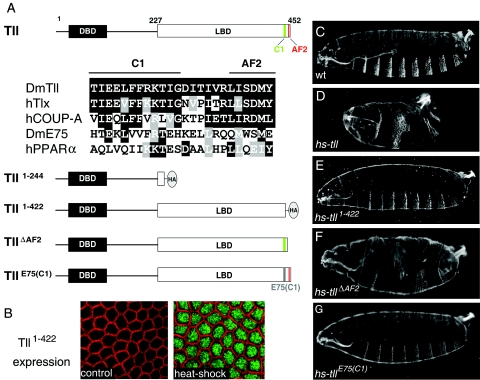
The C-terminal portion of the Tll LBD is essential for patterning activity in the early embryo. (A) Diagram of the Tll protein indicating the DNA-binding domain (DBD) and LBD and the presence of two C-terminal motifs, AF2 and C1. Numbers indicate amino acid positions. An alignment of C1 and AF2 sequences from several nuclear receptors is shown below; identical and similar residues are boxed in black and gray, respectively. The AF2 motif (also known as helix 12) conforms to the consensus ΦΦX(E/D)ΦΦ, where “Φ” denotes a hydrophobic residue and “X” is any amino acid. The C1 motif is well conserved in the human Tll ortholog, Tlx, and in the human COUP-TFA receptor, which, like Tll, belongs to subfamily 2 of nuclear receptors. In contrast, this motif has diverged in two distant receptors belonging to subfamily 1, Drosophila E75 and human peroxisome proliferator-activated receptor α. A diagram of four Tll derivatives with mutations in their LBDs is also shown. “HA” marks the positions of this epitope tag in the Tll1-244 and Tll1-422 derivatives. The E75 sequence replacing the Tll C1 motif in the TllE75(C1) construct is gray. (B) Immunodetection of Tll1-422 protein following heat shock induction using an anti-HA antibody. Detailed surface views of non-heat-shocked (control, left) and heat-shocked (right) hs-tll1-422 blastoderm embryos are shown. Tll1-422 protein (green) accumulates efficiently in the nucleus after heat shock. The cortical actin associated with the growing plasma membranes appears labeled with rhodamine-phalloidin (red). (C) Cuticle of a wild-type (wt) embryo. (D to G) Representative cuticles of heat-shocked hs-tll (D), hs-tll1-422 (E), hs-tllΔAF2 (F), and hs-tllE75(C1) (G) embryos. Note that Tll1-422 and TllE75(C1) are inactive in the assay, whereas TllΔAF2 displays considerable patterning activity.