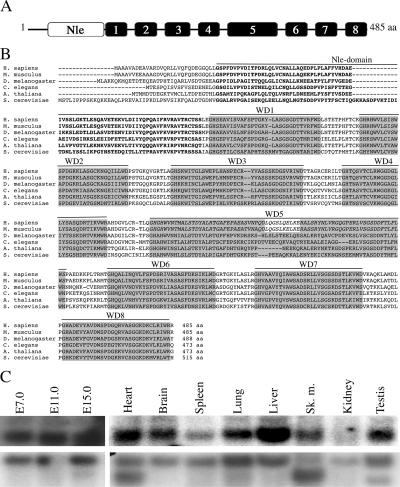
FIG. 1.
Structure and amino acid alignments of mNle protein and expression pattern of mNle transcripts. (A) Schematic representation of the mNle protein containing a single Nle domain (white box) at its amino-terminal region and eight WD40 domains (gray boxes). (B) Alignments of sequences of Nle orthologs by clustalW. Using the website at http://bmerc-www.bu.edu/wdrepeat to search for WD40 domains, eight domains were predicted for Nle in Saccharomyces, Xenopus, and Drosophila, whereas nine domains were predicted for mouse and human Nle proteins. Based on amino acid sequence comparisons and on the fact that, in mouse and human, the fifth domain does not end by a consensus WD motif and that the sixth domain does not begin by a consensus GH sequence, we propose that the fifth and sixth predicted WD40 domains of the human and mouse Nle proteins correspond to a single WD40 domain (in italics), as predicted for other species. GenBank/EMBL accession numbers for the Nle orthologous sequences are as follows: Homo sapiens, NP_060566; Mus musculus, NP_663406; D. melanogaster, NP_477294.1; C. elegans, NP_493745; Arabidopsis thaliana, NP_200094.1; and S. cerevisiae, NP_009997. Amino acids corresponding to the Nle domain are in boldface type. WD40 repeat domains are highlighted in gray boxes. The numbers of amino acids (aa) are indicated at the end of the sequences. (C) Northern blot analysis of poly(A)+ mRNAs of embryos at E7.0 to E15.0 and various adult tissues hybridized with an mNle-specific probe (upper panel) or a β-actin probe (lower panel).