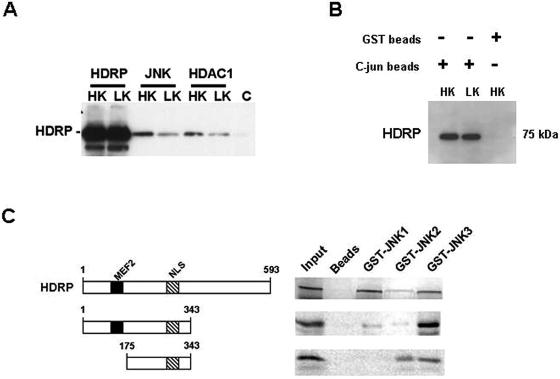
FIG. 11.
Association of HDRP with JNK, HDAC1, and c-Jun complexes. (A) JNK (JNK1, -2, and -3), HDAC1, and HDRP were immunoprecipitated from Ad-HDRP-infected neurons treated for 3 h in HK or LK medium. Immunoprecipitates were subjected to Western blotting, and the membrane was probed with c-Myc antibody for the detection of exogenous HDRP. A bead-only control (C) was used to confirm the fidelity of the immunoprecipitation. (B) Truncated c-Jun-GST conjugated to glutathione-Sepharose beads was used to immunoprecipitate exogenous HDRP from Ad-HDRP-infected neuronal lysates that were treated with HK or LK medium for 3 h. Western blotting was performed on the immunoprecipitate using a c-Myc antibody to detect exogenous HDRP. The specificity of the interaction was confirmed using glutathione-Sepharose beads in place of the c-Jun beads. (C) For in vitro binding assays, p54 isoforms of JNK1-, JNK2-, and JNK3-GST fusion proteins were conjugated to glutathione-Sepharose beads and incubated with [35S]methionine-labeled HDRP and HDRP N (1-343) and N1 (175-343) domains. Associated proteins were resolved by SDS-polyacrylamide gel electrophoresis followed by autoradiography. 35S-labeled HDRP and the N and N1 domains interacted with immobilized GST-JNK1, -2, and -3 but not with the negative control, glutathione-Sepharose resin (beads). The input lane represents 10% of the [35S]methionine-labeled protein that was added to each reaction and serves as a reference marker.