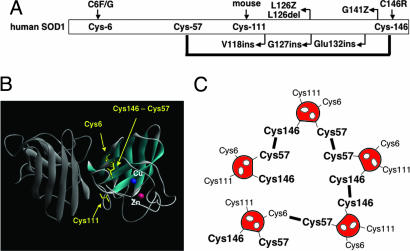
Fig. 5.
Importance of SOD1 Cys residues in ALS-associated mutations and disulfide-linked protein aggregation. (A) Cys-6 and -146 have been shown to link with fALS. Several truncated mutations in SOD1 lack Cys-146. Mouse SOD1 does not have Cys residue at position 111 but has serine. (B) Crystal structure of holoform of human SOD1 (1HL5). In the active form of SOD1, Cys-57 and -146 form the intramolecular disulfide bond, whereas Cys-6 and -111 are intact. (C) Cys-57 and -146 exhibit higher reactivity to form disulfide compared with Cys-6 and -111 (Fig. 3). Although Cys-146 is necessary for extensive disulfide-linked multimerization (Fig. 4), several pairs of Cys residues, i.e., Cys-57-Cys-57, Cys-57-Cys-146, and Cys-146-Cys-146, are possible. Note, nonconserved Cys residues also may contribute to the formation of disulfide-linked multimers.