Abstract
Objectives. We compared patterns of mortality among men with prostate cancer at 2 Department of Veterans Affairs (VA) and 2 private-sector hospitals in the Chicago area.
Methods. Mortality rates for 864 cases diagnosed between 1986 and 1990 were estimated using Cox proportional hazards models that incorporated age; income; cancer stage, differentiation, and treatments; and baseline comorbidity.
Results. Race tended to associate with all-cause mortality irrespective of health care setting (Blacks vs Whites: hazard rate ratio [HRR] = 1.68 [95% confidence interval (CI) = 1.06, 2.67]; P < .001 in the private sector; HRR = 1.50 [95% CI = 0.94, 2.38]; P = .088 in the VA). However, comorbidity determined risk in the VA, whereas age and income predicted risk in the private sector.
Conclusions. Determinants of all-cause mortality in men with prostate cancer vary according to health care setting.
Prostate cancer remains the most commonly occurring cancer and the second most common cause of cancer-related mortality among US males.1 The American Cancer Society has projected that 220 900 new cases and 28 900 prostate cancer–related deaths will occur in the year 2003 alone.2 One of the most striking features of the disease in the United States is the marked and persistent racial/ethnic disparity in incidence and mortality. Compared with their White counterparts, Black men with prostate cancer tend to have shorter survival times even after we accounted for age, stage, and histological differences at the time of diagnosis.3 In the United States, race/ethnicity is a proxy for social and demographic forces that influence health care access, utilization, and treatment patterns contributing to group differences in outcomes of chronic disease in general. Therefore, the most prominent hypotheses put forth to explain US racial disparities in prostate cancer have been patient and provider behavioral barriers and health care system access failure.4–8
The Veterans Health Administration (VHA), the largest integrated health care system in the United States, attempts to equalize access by significantly reducing financial barriers to health-related services.9 This setting seems particularly well suited to an examination of the impact of this parity in access on racial disparities in survival among men with prostate cancer. Therefore, the primary focus of this study was to compare the relation of race and cause-specific mortality in the VHA health care system with that in the private sector. A secondary purpose was to compare the determinants of mortality among prostate cancer patients in these health care settings to shed light on social and clinical characteristics that may contribute to or modify racial differences in mortality.
METHODS
Study Design and Patient Population
We used a retrospective cohort design. The cohort consisted of all cases of adenocarcinoma of the prostate (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 187.010) diagnosed among Black and White men at 2 Department of Veterans Affairs (VA) hospitals and 2 private university medical centers between January 1, 1986, and December 31, 1990. Of the 1163 cases of adenocarcinoma of the prostate originally identified in the tumor registry of each hospital, 1007 (87%) had medical records available for detailed review.
Baseline Characteristics
Inpatient and outpatient medical records were abstracted on-site by 2 trained reviewers. The data abstracted from each record included demographics (name, race, date of birth, Social Security number, zip code), tumor characteristics (stage, differentiation, Gleason sum), processes of diagnosis and management (indication for diagnostic evaluation, method of diagnosis, clinical date of diagnosis, pathological date of diagnosis, metastatic evaluation, first-course treatments), comorbidities present at the time of diagnosis, and subsequent health outcomes (disease recurrence/progression date and location, vital status, and cancer status).11–13 Staging was based on all of the evidence available in the original patient record, and assignments were made according to the American Joint Committee on Cancer TNM (tumor, nodes, metastasis) staging system.11 Comorbid conditions present at the time of diagnosis was measured with the index developed by Charlson and others.14
Agreement between the 2 reviewers was monitored for race, clinical and pathological diagnosis date, tumor differentiation, stage, treatment modality, treatment date, comorbidities, Charlson scoring, last contact date, and vital status in a 20% random sample of all records reviewed. The level of agreement was high (κ = 0.95–0.98).
Exclusions
Ninety of the cases were excluded because cancers were T1a-stage lesions, which are believed to be clinically insignificant, and 53 others were excluded because of incomplete data. This exclusion left 864 records (385 in the VA, 479 in the private sector) for analysis. Blacks made up 38.8% of the analytic cohort, and 64% of Black patients were diagnosed at one of the VA hospitals. Of the records not available for detailed review, 42% were for Black patients.
Outcomes and Their Ascertainment
Follow-up ended on December 31, 2000, with death from prostate cancer and death from other causes as the primary outcomes of interest. The tumor registry at each hospital was our primary source for vital status ascertainment. We also conducted multiple searches of the National Death Index of the National Center for Health Statistics and the VA Beneficiary Identification and Record Locator System over a 24-month period beginning January 1, 2000. Causes of death were established on the basis of death certificate review performed by an independent physician–reviewer blinded to the study’s hypotheses. Causes of death were coded according to ICD-9-CM.10
Statistical Methods
All analyses were performed for the entire cohort and within each health care setting. Baseline demographic and clinical characteristics of Blacks were compared with those of Whites using the 2-sample t test for continuous traits and χ2 analysis for categorical ones. Kaplan–Meier survival distributions were computed for subgroups of men defined by race (Black, White), tumor differentiation (well, moderate, poor), stage (local/regional, distant), and Charlson score (0–1, 2–3, ≥ 4), and these distributions were compared using the Mantel–Haenszel statistic.15
Local-/regional-stage tumors are defined as those confined to the prostate gland or extraprostatic extension, with or without regional lymph node involvement (grouping = T1b–3 any, N0–3, M0). Distant-stage tumors are defined as distant metastases, including disease that has spread beyond the pelvis to areas including bones, liver, lungs, and brain (grouping = T4, NX–0, M0, or T any, N any, M1). In this study, local- and regional-stage cases were combined because they would be candidates for aggressive curative therapy. Unstaged cases were combined with distant cases because their respective survival distributions were not significantly different (χ21 = 0.30; P = .58). The 3 histological subgroups used in our analyses—well, moderately, and poorly differentiated tumors—generally corresponded to Gleason sums of 2–4, 5–6, and 7–10, respectively. Charlson comorbidity scores were available for all but 2.6% of patients. These values were imputed by a multiple regression imputation method in which the available comorbidity score was regressed on age at diagnosis, race, hospital of origin, stage, differentiation, and comorbid diseases or conditions (coronary artery disease, hypertension, tobacco and alcohol usage).
A stratified Cox proportional hazards regression model was used to account for the baseline hazard associated with the hospital where the case originated.16 The regression model included age, race (Black, White), Charlson comorbidity score, tumor differentiation (well, moderately, poorly differentiated), stage (local/regional, distant), first-course treatment (surgery, radiation, diethylstilbestrol, castration, observation) and mean household income per capita by race for zip code of residence according to the 1990 US Census.17,18 Potential interactions between comorbidity with stage and race were evaluated by including comorbidity-by-stage and comorbidity-by-race terms in the regression model. Ninety-five percent CIs for hazard rates of death from any cause for Blacks relative to Whites by comorbidity level (Charlson scores = 0, 1, 2, 3, 4, and ≥ 5) were computed with a bias-corrected bootstrap approach based on 2500 bootstrap samples.19 Different regression models were considered before we reached the final models. The proportional hazards assumption in each was tested with time-dependent coefficients and Schoenfeld residuals.20 No violations of the proportional hazards assumptions were observed (P = .23 to .97). The statistical analyses were performed with Stata Version 7.0.21
RESULTS
Baseline Characteristics
Table 1 ▶ shows a comparison of baseline sociodemographic and clinical characteristics of Blacks and Whites in both health care systems. Blacks tended to reside in areas of lower household income per capita (mean income = $8508 vs $15 782 for VA patients, $9852 vs $20 689 for private-sector patients; P < .001), were significantly more likely to present with distant-stage disease (37.1% vs 29.1% for VA patients, 48.7% vs 23.3% for private-sector patients), and were significantly less likely to receive aggressive curative therapy for local-/regional-stage prostate cancer (62.9% vs 82% for VA patients, 73.3% vs 85.6% for private-sector patients). Within each system, Blacks and Whites had similar levels of comorbidity, but the levels of VA patients appeared to be greater than those of patients in the private sector (mean Charlson score = 1.3 for VA patients vs 2.2 for private-sector patients; P < .001). In the private sector, Blacks were older (mean = 70.7 years vs 68.9 years; P = .044), had a higher crude all-cause mortality rate (72.7% vs 48.9%; P < .001), and had shorter survival times (mean = 4.1 years vs 6.1 years; P < .001) compared with their White counterparts. However, significant racial differences in age, crude all-cause mortality, and survival times were not observed in the VA.
TABLE 1—
Baseline Characteristics of Sample Populations: 1986–1990
| Department of Veterans Affairs (n = 385) | Private Sector (n = 479) | |||||
| Blacks | Whites | P | Blacks | Whites | P | |
| Sample size, n (%) | 210 (55) | 175 (45) | . . . | 117 (24) | 362 (76) | . . . |
| Mean age, y (SE) | 68.5 (0.5) | 68.5 (0.5) | .967 | 70.7 (0.9) | 68.9 (0.4) | .044 |
| Mean household income per capita, $ (SE) | 8508 (199) | 15782 (390) | <.001 | 9852 (250) | 20689 (512) | <.001 |
| Differentiation, n (%) | .025 | .033 | ||||
| Well | 63 (30.0) | 76 (43.4) | . . . | 17 (14.5) | 79 (21.8) | . . . |
| Moderate | 70 (33.3) | 46 (26.3) | . . . | 33 (28.2) | 125 (34.5) | . . . |
| Poor | 77 (36.8) | 53 (30.3) | . . . | 67 (57.3) | 158 (43.7) | . . . |
| Stage, n (%) | .044 | <.001 | ||||
| Local | 92 (43.8) | 99 (56.6) | . . . | 40 (34.2) | 206 (56.9) | . . . |
| Regional | 40 (19.1) | 25 (14.3) | . . . | 20 (17.1) | 72 (19.9) | . . . |
| Distant/unstageda | 78 (37.1) | 51 (29.1) | . . . | 57 (48.7) | 84 (23.3) | . . . |
| Surgery and/or radiation for local-/regional-stage cancer, n (%) | 83 (62.9) | 102 (82.2) | <.001 | 44 (73.3) | 238 (85.6) | .033 |
| Mean Charlson score (SE)b | 2.3 (0.2) | 2.1 (0.2) | .234 | 1.5 (0.2) | 1.3 (0.1) | .329 |
| Charlson score group, n (%) | .424 | .710 | ||||
| 0–1 | 94 (44.8) | 89 (50.9) | . . . | 76 (65.0) | 247 (68.2) | . . . |
| 2–3 | 73 (34.8) | 51 (29.1) | . . . | 27 (23.0) | 79 (21.8) | . . . |
| ≥ 4 | 43 (20.5) | 35 (20.0) | . . . | 14 (12.0) | 36 (10.0) | . . . |
| Deaths, n (%) | 137 (65.2) | 108 (61.7) | . . . | 85 (72.7) | 177 (48.9) | . . . |
| Time to death mean, y (SE) | 5.3 (0.2) | 5.7 (0.3) | .293 | 4.1 (0.3) | 6.1 (0.2) | <.001 |
aPresumed to be distant stage by the treating physician but not staged for 3 Black patients and 2 White patients.
bScores ranged from 0 to 14.
Overall Survival by Race and Comorbidity Level
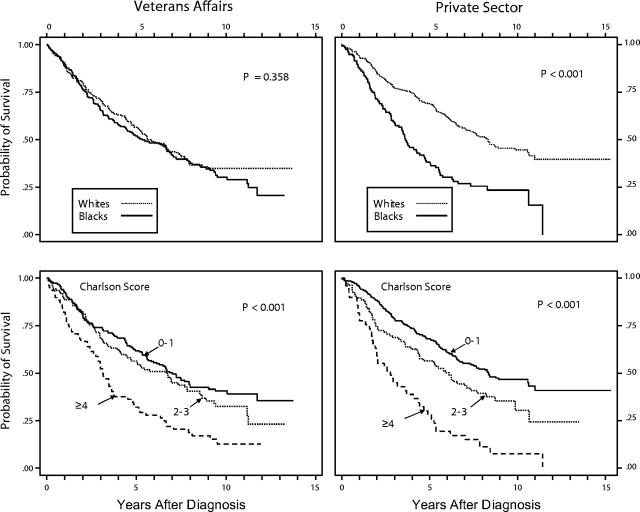
Figure 1 ▶ presents Kaplan–Meier survival distributions by race and by comorbidity level. Blacks in the private sector fared significantly worse than did Whites, with a median crude survival time of approximately 3.5 years for Blacks and 8.2 years for Whites (χ2 = 37.6; P < .001). However, no racial differences in survival were found in the VA, where the median survival for both groups was 5.7 years. Results by comorbidity level were more nuanced. In the private sector, Charlson score groupings of 0–1, 2–3, and 4 or higher yielded significantly distinct survival distributions (median survival = 8.3 years, 5.9 years, and 2.7 years, respectively; χ2 = 53.3; P < .001). However, stratification of VA cases in the same manner did not result in as great a contrast in overall survival. Median survival distribution for Charlson score groupings of 0–1, 2–3, and 4 or higher was 7.0 years, 6.8 years, and 3.2 years, respectively (χ2 = 25.4; P < .001; for 0–1 vs 2–3 only, χ2 = 1.44; P < .230).
FIGURE 1—
Kaplan–Meier survival distributions by race and by comorbidity level.
Predictors of Mortality
Predictors of all-cause and cause-specific mortality in each health care setting are shown in Table 2 ▶. In the VA, tumor differentiation (poor vs well), stage at diagnosis (local/regional vs distant) and Charlson score predicted risk of death from any cause (hazard rate ratios [HRRs] = 1.68 [95% confidence interval (CI) = 1.19, 2.38], 0.26 [95% CI = 0.15, 0.44], and 1.16 [95% CI = 1.04, 1.29], respectively; P < .001 to .003). The relations of tumor characteristics (stage, differentiation) and all-cause mortality appeared to be attributable to significant associations with risk of death from prostate cancer, whereas the relation between Charlson score and all-cause mortality was explained by its significant positive association with risk of death from other causes (HRR = 1.36 [95% CI = 1.18, 1.56] per unit increase; P < .001). Blacks had a 25% to 50% excess in mortality relative to Whites, depending on the cause of death, but these differences did not reach statistical significance.
TABLE 2—
Relative Hazard Ratios for Death, by Cause,a for 864 Black and White Men Diagnosed with Prostate Cancer: 2 Veterans Affairs and 2 Private Sector Hospitals, Chicago Area, 1986–1990
| Department of Veterans Affairs (n = 385) | Private Sector (n = 479) | |||||||||||
| All Causes (n = 245) | Prostate Cancer (n = 102) | Other (n = 143) | All Causes (n = 262) | Prostate Cancer (n = 113) | Other (n = 149) | |||||||
| HRR (95% CI) | Pb | HRR (95% CI) | P | HRR (95% CI) | P | HRR (95% CI) | P | HRR (95% CI) | P | HRR (95% CI) | P | |
| Race (Black vs White) | 1.50 (0.94, 2.38) | .088 | 1.36 (0.62, 2.96) | . . . | 1.25 (0.66, 2.35) | . . . | 1.68 (1.06, 2.67) | < .001 | 2.68 (1.36, 5.27) | < .001 | 1.03 (0.54, 1.97) | . . . |
| Age (5-y increments) | 1.07 (0.97, 1.17) | . . . | 1.03 (0.90, 1.19) | . . . | 1.06 (0.93, 1.21) | . . . | 1.17 (1.07, 1.28) | .001 | 0.99 (0.87, 1.12) | . . . | 1.40 (1.23, 1.60) | < .001 |
| Income (per $1000)c | 1.00 (0.97, 1.05) | . . . | 1.02 (0.96, 1.08) | . . . | 1.00 (0.96, 1.04) | . . . | 0.98 (0.96, 0.99) | .025 | 1.00 (0.98, 1.02) | . . . | 0.94 (0.91, 0.97) | < .001 |
| Differentiation (vs well) | ||||||||||||
| Moderate | 1.24 (0.88, 1.76) | . . . | 2.23 (1.15, 4.29) | .017 | 0.91 (0.59, 1.41) | . . . | 1.54 (1.02, 2.31) | .040 | 2.49 (1.06, 5.86) | .037 | 1.18 (0.73, 1.90) | . . . |
| Poor | 1.68 (1.19, 2.38) | .003 | 3.41 (1.79, 6.47) | < .001 | 1.28 (0.82, 1.98) | . . . | 1.83 (1.23, 2.73) | .003 | 4.58 (2.06, 10.2) | < .001 | 1.06 (0.65, 1.73) | . . . |
| Stage (local/regional vs distant) | 0.26 (0.15, 0.44) | < .001 | 0.18 (0.08, 0.43) | < .001 | 0.48 (0.23, 1.01) | .052 | 0.31 (0.21, 0.48) | < .001 | 0.19 (0.10, 0.36) | < .001 | 0.56 (0.31, 1.02) | .056 |
| Treatment | ||||||||||||
| Surgery | 0.66 (0.42, 1.04) | .074 | 0.40 (0.17, 0.96) | .041 | 0.72 (0.41, 1.25) | . . . | 0.49 (0.31, 0.77) | .001 | 0.45 (0.21, 0.97) | .041 | 0.54 (0.30, 0.96) | .035 |
| Radiation | 1.00 (0.67, 1.48) | . . . | 1.25 (0.83, 1.30) | . . . | 0.90 (0.53, 1.52) | . . . | 1.18 (0.84, 1.68) | . . . | 1.80 (1.09, 2.98) | .022 | 0.92 (0.58, 1.45) | . . . |
| DES | 1.09 (0.61, 1.95) | . . . | 1.81 (0.84, 3.88) | . . . | 0.50 (0.18, 1.42) | . . . | 1.88 (0.98, 3.60) | .057 | 1.25 (0.44, 3.60) | . . . | 3.08 (1.34, 7.08) | .008 |
| Castrationd | 0.89 (0.55, 1.43) | . . . | 1.14 (0.57, 2.29) | . . . | 0.72 (0.37, 1.41) | . . . | 1.38 (0.94, 2.04) | . . . | 1.91 (1.10, 3.31) | .021 | 1.14 (0.63, 2.04) | . . . |
| Observation | 1.51 (0.90, 2.55) | . . . | 0.47 (0.14, 1.66) | . . . | 2.04 (1.10, 3.79) | .024 | 1.14 (0.62, 2.09) | . . . | 0.61 (0.14, 2.21) | . . . | 1.26 (0.62, 2.55) | . . . |
| Charlson scoree | 1.16 (1.04, 1.29) | .008 | 0.98 (0.80, 1.20) | . . . | 1.36 (1.18, 1.56) | < .001 | 1.01 (0.89, 1.15) | . . . | 1.06 (0.89, 1.26) | . . . | 1.02 (0.84, 1.22) | . . . |
Note. HRR = hazard rate ratio; CI = confidence interval; DES = diethylstilbestrol.
aRelative hazard rations were calculated using a stratified Cox proportional hazards regression model, which accounted for the baseline hazard associated with the hospital where the case originated and is specified as follows: λ = λ0(t) exp(β1race + β2age + β3moderate diff + β4poorly diff + β5loc_regstg + β6surgery + β7radiation + β8DES + β9castration + β10observation + β11score + β12black*score + β13loc_regstg*score).
bOnly P values below .10 are reported in this table.
cMean household income per capita for zip code of residence at time of diagnosis.
dOrchiectomy or leuprolide and/or flutamide.
eScores ranged from 0 to 14.
By contrast, race was a significant predictor of all-cause mortality in the private sector, even after we accounted for sociodemographic and clinical differences at baseline (Blacks vs Whites: HRR = 1.68 [95% CI = 1.06, 2.67]; P < .001). This association was attributable to racial disparities in risk of death from prostate cancer (Blacks vs Whites: HRR = 2.68 [95% CI = 1.36, 5.27]; P < .001) but not from other causes. Increasing age (in 5-year increments) was also associated with increasing risk of death from any cause (HRR = 1.17 [95% CI = 1.07,1.28]; P = .001), whereas increasing income (per $1000 per capita for household) appeared to be protective (HRR = 0.98 [95% CI = 0.96, 0.99]; P = .025). In both cases, significant associations with risk of death from other causes explained the relation with all-cause mortality (HRRs = 1.40 [95% CI = 1.23, 1.60] and 0.94 [95% CI = 0.91, 0.97] for age and income, respectively; P < .001). Similarly, the association of cancer stage and differentiation with risk of death from prostate cancer accounted for their independent relation with all-cause mortality. However, comorbidity as measured with the Charlson index did not predict mortality from any cause in the private sector after adjustment for race, age, income, and histological differences at baseline.
Racial Differences in Survival in Relation to Initial Comorbidity
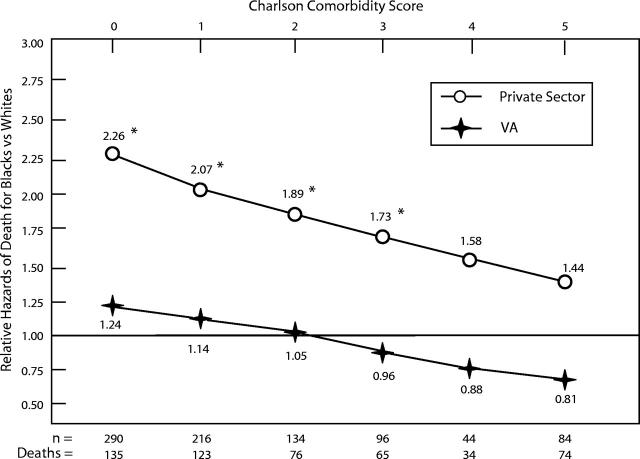
Figure 2 ▶ shows all-cause mortality rates for Blacks relative to Whites by Charlson score after adjustment for age, stage, tumor differentiation, and treatment. The relative excess all-cause mortality experienced by Blacks was inversely related to initial comorbidity. This association was more pronounced in the private sector, where excess mortality rates among Blacks relative to Whites tended to be statistically significant (HRRs = 2.26 [95% CI = 1.40, 3.45], 2.07 [95% CI = 1.45, 2.97], 1.89 [95% CI = 1.31, 2.85], 1.73 [95% CI = 1.07, 3.07], 1.58 [95% CI = 0.84, 3.42], and 1.44 [95% CI = 0.65, 3.91], respectively, for Charlson scores 0, 1, 2, 3, 4, and ≥ 5; P < .05). We found a parallel trend in the VA system, but none of the relative hazard estimates reached statistical significance (HRRs = 1.24 [95% CI = 0.82, 1.92], 1.14 [95% CI = 0.80, 1.59], 1.05 [95% CI = 0.75, 1.37], 0.96 [95% CI = 0.67, 1.26], 0.88 [95% CI = 0.56, 1.20], and 0.81 [95% CI = 0.45, 1.20], respectively).
FIGURE 2—
Bootstrap confidence intervals for all-cause mortality rates of Blacks relative to Whites, by Charlson comorbidity score.
Note. VA = Department of Veterans Affairs. Estimates are adjusted for age, tumor stage, tumor differentiation, and treatment.
*P < .05.
DISCUSSION
With few exceptions, evidence of racial/ethnic disparities in health and health care is fairly consistent across a broad range of illnesses and health care services.4 Some studies suggest that these disparities are either reduced or eliminated in the health care systems of the US Department of Defense and the VA.22–26 This has been observed more consistently in studies of the Department of Defense system, which ensures universal access, than in the VA system, which significantly reduces the financial barriers to health care for veterans. Consequently, investigators often make the following inferences regarding racial differences in health outcomes detected in such health care systems: (1) their absence is likely the result of reducing the economic pressures associated with chronic disease control and health maintenance; and (2) their persistence reflects residual confounding due to differences in access to health services, utilization patterns, and processes of care or differences in disease severity over time. Indeed, racial/ethnic disparities in health status and health care, which usually are associated with socioeconomic differences, tend to remain even after control for socioeconomic status and other health care access–related factors.27,28 This pattern may be the case in our cohort of prostate cancer patients.
After adjustment for age, income, tumor characteristics, treatment, and comorbidities at baseline, all-cause mortality rates among Blacks relative to Whites were 1.68 (95% CI = 1.06, 2.67; P < .001) in the private sector and 1.50 (95% CI = 0.94, 2.38; P < .088) in the VA. Because the rate ratio for race in the VA subset would probably reach statistical significance in a larger sample, application of a statistically based dichotomous interpretation of significance (Neyman–Pearson hypothesis testing) could misinterpret this result.29 Therefore, we conclude that at least a trend toward excess all-cause mortality among Blacks relative to Whites existed in the VA hospitals.
The relationship between race and disparities in survival among men with prostate cancer is well established in the private sector.1 However, findings within the VA and other health care settings assumed to offer equal access to care are mixed.30–33 Our results differ from those findings in that we attempted to account for the simultaneous contributions of age, prostate cancer stage and histopathological severity, treatments, comorbidity burden, and income. Moreover, adjustment for these factors narrowed racial differences in mortality in the private sector but exposed them in the VA.
Clinical factors also predicted mortality in our cohort, but with different degrees of cause specificity. For example, tumor differentiation predicted risk of death from prostate cancer but not from other causes. On the other hand, early-stage diagnosis (i.e., cancer confined to the prostate gland or extraprostatic extension with or without ipsilateral lymph node involvement) not only correlated with a lower risk of death from prostate cancer but also tended to correlate inversely with risk of death from other causes (for VA patients: HRR = 0.48 [95% CI = 0.23, 1.01]; P = .052; for private-sector patients: HRR = 0.54 [95% CI = 0.30, 0.96]; P = .056). Also contrary to expectation was the lack of an association between comorbidity at time of diagnosis and subsequent mortality in the private sector. The absence of racial disparities in risk of death from other causes in the private sector is also inconsistent with observations made by many others.34–36 However, excess all-cause mortality of Blacks in the private-sector subset seemed to derive from risk of death from prostate cancer. This pattern suggests that biases in cause-of-death attribution may have partly accounted for the lack of association of race with death from other causes.
What do these data say about determinants of mortality and potential sources of racial disparities in survival after a diagnosis of prostate cancer? First, determinants of all-cause and cause-specific mortality vary according to health care setting, with sociodemographic factors such as age and income playing a role in the private sector but not in the VA system. Second, the association between certain aspects of prostate cancer diagnosis and management with mortality from other causes suggests the presence of more general patient, provider, and health care system characteristics that impact health status and its prognosis in general. These characteristics, in turn, can contribute to or modify racial disparities in survival. For example, diagnosis of prostate cancer at a clinically early stage (i.e., local or regional) tended to be associated with a lowered risk of death not only from prostate cancer but also from other causes. However, Blacks were significantly more likely to present with distant-stage disease. Undergoing surgery for prostate cancer also tended to correlate with a lowered risk of death from other causes. However, Blacks in our cohort were significantly less likely than Whites to receive surgery or other aggressive curative therapy for clinically early-stage disease. Such racial differences in stage at presentation and aggressive primary therapy where indicated have been reported by others.8,11 Factors that lead to diagnosis and treatment differences in prostate cancer may affect the recognition and prognosis of other health conditions. These include (1) racial/ethnic differences in patient preferences and care-seeking behaviors and attitudes,37,38 (2) provider bias against minorities and greater clinical uncertainty in interactions with minority patients, resulting in differences in disease detection and treatment,35,39–43 and (3) structural arrangements for services (geography, centralization of services) that fragment the delivery of care.44–47
Finally, the effect of race on all-cause mortality varied by comorbidity level at diagnosis in each health care setting. This variation is indicated by the inverse relation between relative hazards of death (Blacks vs Whites) and Charlson score, as shown in Figure 2 ▶. The coefficient for the race-by-comorbidity interaction term incorporated into our Cox proportional hazards model was statistically significant in the cohort overall (P < .001). These observations suggest either modification of the prognostic significance of racial factors by comorbidity or, conversely, modification of the prognostic significance of comorbidity by racial factors. Intrinsic biological differences between Blacks and Whites seem an unlikely explanation, especially because the association between relative all-cause mortality rates and Charlson score is inverted. Therefore, modification of the prognostic significance of comorbidity by racial factors seems the more likely hypothesis.
Among men with local-/regional-stage prostate cancer, these race-specific estimates for the risk of death from any cause per unit increase in Charlson score were observed after adjustment for age, tumor differentiation, and treatments: for VA patients (Whites vs Blacks): HRR (95% CI) = 1.20 (1.09, 1.43) vs 1.10 (1.00, 1.22); for private sector patients: HRR (95% CI) = 1.28 (1.19, 1.51) vs 1.17 (0.97, 1.56). Hence, comorbidity at the time of diagnosis tended to be less predictive of all-cause mortality among Blacks. This trend may reflect racial differences in the prognostic performance of the Charlson index. Possible mechanisms for such differential performance include less precise ranking of the severity of comorbidities among Blacks and a lower sensitivity in detecting prognostically important conditions (which may be more likely to go unrecognized or be less fully characterized in Blacks compared with Whites44,48,49). In light of these possibilities, the results shown in Figure 2 ▶ suggest that absence of a significant preexisting medical diagnosis is a risk factor for excess all-cause mortality among Black men with prostate cancer.
Limitations
Prostate-specific antigen (PSA) is an important marker of prostate cancer disease activity and prognosis.50 However, the results of PSA testing were not available for approximately 15% of our cohort, and levels were not accounted for in our analyses. Racial and ethnic minorities are more likely than Whites to be enrolled in “lower-end” health plans. Such plans are characterized by higher per-capita resource constraints and stricter limits on covered services that may affect health care utilization.46 However, data on payment sources in the private sector were not available for this analysis.
In conclusion, determinants of all-cause mortality in men with prostate cancer vary between the Veterans Affairs and private-sector health care systems. More detailed analysis of the sociodemographic and clinical characteristics of patients, provider behavior, and structural arrangements for services in each health care setting in relation to cause-specific mortality should provide important insights into the causes of racial/ethnic disparities in survival in this population and into strategies to eliminate these disparities.
Acknowledgments
This research was supported by the Robert Wood Johnson Foundation and the Department of Veterans Affairs.
Human Participant Protection Appropriate institutional review board approval was obtained at each hospital.
Contributors V. L. Freeman, R. Durazo-Arvizu, and L. C. Keys designed and implemented the study, including developing the hypotheses, conducting sample size and statistical power analyses, designing the data collection instrument, supervising data collection, and performing quality controls. V. L. Freeman and R. Durazo-Arvizu conducted the data analyses. All of the authors interpreted the data and drafted or revised the article.
Peer Reviewed
References
- 1.Stanford JL, Stephenson RA, Coyle LM, eds. Prostate Cancer Trends, 1973–1995. Bethesda, Md: SEER Program, National Cancer Institute; 1999. NIH publication 99-4543.
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. [DOI] [PubMed] [Google Scholar]
- 3.Merrill RM, Brawley OW. Prostate cancer incidence and mortality rates among white and black men. Epidemiology. 1997;8:126–131. [DOI] [PubMed] [Google Scholar]
- 4.Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2002. [PubMed]
- 5.Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- 6.Jepson C, Kessler LG, Portnoy B, Gibbs T. Black–White differences in cancer prevention knowledge and behavior. Am J Public Health. 1991;81:501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers RE, Wolf TA, Balshem AM, Ross EA, Chodak GW. Receptivity of African-American men to prostate cancer screening. Urology. 1994;43:480–487. [DOI] [PubMed] [Google Scholar]
- 8.Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13:93–100. [DOI] [PubMed] [Google Scholar]
- 9.Kizer KW. Transforming the veterans health care system—the new VA. JAMA. 1996;275:1069–1074.8601910 [Google Scholar]
- 10.International Classification of Diseases, Ninth Revision, Clinical Modification. Hyattsville, Md: National Center for Health Statistics; 1980. DHHS publication PHS 80-1260.
- 11.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 5th ed. Philadelphia, Pa: Lippincott-Raven; 1997.
- 12.Schmidt JD, Mettlin CJ, Natarajan N, et al. Trends in patterns of care for prostatic cancer, 1974–1983: results of surveys by the American College of Surgeons. J Urol. 1987;136:416–421. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan N, Murphy GP, Mettlin C. Prostate cancer in blacks: an update from the American College of Surgeons’ pattern of care studies. J Surg Oncol. 1989;40:232–236. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Miller RG. Survival Analysis. New York, NY: John Wiley & Sons; 1981.
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980.
- 17.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. 1990 Census of Population—Social and Economic Characteristics. Washington, DC: US Dept of Commerce; 1993. Publication CP-2.
- 19.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hill; 1993.
- 20.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000.
- 21.Stata [computer program]. Version 7.0. College Station, Tex: Stata Corp; 2001.
- 22.Optenberg A, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274:1599–1605. [PubMed] [Google Scholar]
- 23.Selim AJ, Fincke G, Ren XS, et al. Racial differences in the use of lumbar spine radiographs: results from the Veterans Health Study. Spine. 2001;26:1364–1369. [DOI] [PubMed] [Google Scholar]
- 24.Dominitz JA, Samsa GP, Landsman P, Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82:2312–2320. [DOI] [PubMed] [Google Scholar]
- 25.Horner RD, Oddone EZ, Stechuchak KM, et al. Racial variations in postoperative outcomes of carotid endarterectomy: evidence from the Veterans Affairs National Surgical Quality Improvement Program. Med Care. 2002;40(1 suppl):I35–I43. [PubMed] [Google Scholar]
- 26.Petersen LA, Wright SM, Peterson ED, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Med Care. 2002;40(1 suppl):I86–I96. [DOI] [PubMed] [Google Scholar]
- 27.Mayberry RM, Mili F, Ofili E. Racial differences in access to medical care. Med Care Res Rev. 2000;57(suppl 1):108–145. [DOI] [PubMed] [Google Scholar]
- 28.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2000;135:352–366. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, eds. Modern Epidemiology. 2nd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 1998:184–189.
- 30.Powell IJ, Schwartz K, Hussain M. Removal of the financial barrier to health care: does it impact on prostate cancer at presentation and survival? A comparative study between black and white men in a Veterans Affairs system. Urology. 1995;46:825–830. [DOI] [PubMed] [Google Scholar]
- 31.Wilt TJ, Cowper DC, Gammack JK, Going DR, Nugent S, Borowsky SJ. An evaluation of radical prostatectomy at Veterans Affairs Medical Centers: time trends and geographic variation in utilization and outcomes. Med Care. 1999;37:1046–1056. [DOI] [PubMed] [Google Scholar]
- 32.Freedland SJ, Amling CL, Dorey F, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002;60:670–674. [DOI] [PubMed] [Google Scholar]
- 33.Freedland SJ, Jalkut M, Dorey F, Sutter ME, Aronson WJ. Race is not an independent predictor of biochemical recurrence after radical prostatectomy in an equal access medical center. Urology. 2000;56:87–91. [DOI] [PubMed] [Google Scholar]
- 34.Agency for Healthcare Research and Quality. Addressing racial and ethnic disparities in health care [fact sheet]. Available at: http://www.ahcpr.gov/research/disparit.htm. Accessed July 8, 2003.
- 35.Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285:297–303. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Health Statistics. US Mortality Public Use Data Tape, 1998. Hyattsville, Md: Centers for Disease Control and Prevention; 2000.
- 37.Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35:1220–1224. [DOI] [PubMed] [Google Scholar]
- 38.Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. J Clin Epidemiol. 1997;50:899–901. [DOI] [PubMed] [Google Scholar]
- 39.Okelo S, Taylor AL, Wright JT Jr, Gordon N, Mohan G, Lesnefsky E. Race and the decision to refer for coronary revascularization: the effect of physician awareness of patient ethnicity. J Am Coll Cardiol. 2001;38:698–704. [DOI] [PubMed] [Google Scholar]
- 40.Balsa AI, McGuire TG. Prejudice, clinical uncertainty and stereotyping as sources of health disparities. J Health Econ. 2003;22:89–116. [DOI] [PubMed] [Google Scholar]
- 41.Abreu JM. Conscious and nonconscious African American stereotypes: impact on first impression and diagnostic ratings by therapists. J Consult Clin Psychol. 1999;67:387–393. [DOI] [PubMed] [Google Scholar]
- 42.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Soc Sci Med. 2000;50:813–828. [DOI] [PubMed] [Google Scholar]
- 43.van Ryn M. Research on the provider contribution to race/ethnicity disparities in medical care. Med Care. 2002;40(1 suppl):140–151. [DOI] [PubMed] [Google Scholar]
- 44.Kahn KL, Pearson ML, Harrison ER, et al. Health care for black and poor hospitalized Medicare patients. JAMA. 1994;271:1169–1174. [PubMed] [Google Scholar]
- 45.Phillips KA, Mayer ML, Aday LA. Barriers to care among racial/ethnic groups under managed care. Health Aff. 2000;19:65–75. [DOI] [PubMed] [Google Scholar]
- 46.Bloche MG. Race and discretion in American medicine. Yale J Health Policy Law Ethics. 2001;1:95–131. [PubMed] [Google Scholar]
- 47.Ricketts TC, Randolph R, Howard HA, Pathman D, Carey T. Hospitalization rates as indicators of access to primary care. Health Place. 2001;7:27–38. [DOI] [PubMed] [Google Scholar]
- 48.Harris DR, Andrews R, Elixhauser A. Racial and gender differences in use of procedures for black and white hospitalized patients. Ethn Dis. 1997;7:91–105. [PubMed] [Google Scholar]
- 49.McBean AM, Gornick M. Differences by race in the rates of procedures performed in hospitals for Medicare beneficiaries. Health Care Financ Rev. 1994;15:77–90. [PMC free article] [PubMed] [Google Scholar]
- 50.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–114. [DOI] [PubMed] [Google Scholar]