Figure 5.—
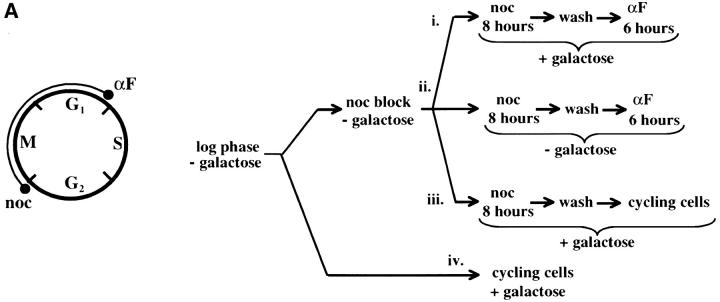
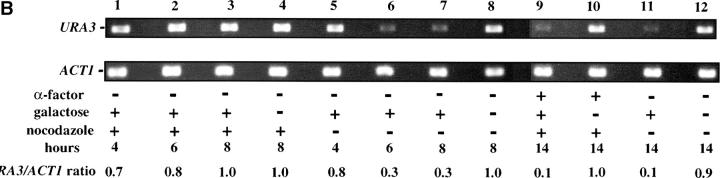
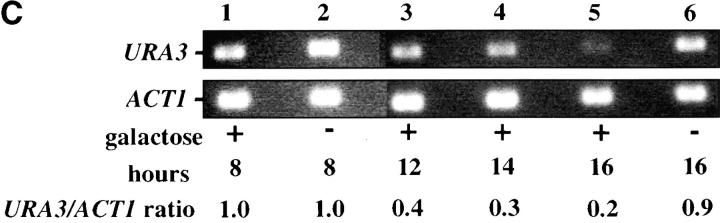
Telomere repression is established in M phase. (A) Experimental design for G2/M–G1 interval. Cultures of strain YSH505 were grown to early log phase in raffinose media. Levels of URA3 message were examined in one experimental and three control cultures. (i) Experimental culture. Following efficient arrest in G2/M phase galactose was added and cultures were incubated in the presence of nocodazole for an additional 8 hr. Cultures were then washed to remove nocodazole and resuspended in galactose media containing α-factor. Cells were then incubated an additional 6 hr. (ii) No galactose control. This culture was treated exactly the same as the experimental culture, but was not induced with galactose. (iii) No α-factor control. This control was treated the same as the experimental culture, except that following the wash step cells were released into galactose media without α-factor. Data for this control are shown in Figure 4C. (iv) Cycling cells control. This culture was induced with galactose but not subjected to cell-cycle blocks. (B) G2/M–G1 interval. RT-PCR was used to determine the levels of URA3 message of cultures grown according to the design described in A. All times listed are in hours following initial addition of galactose to the experimental culture. In the experiment presented galactose was added when 92% of the culture consisted of large-budded cells; following 8 hr of galactose induction large-budded cells composed 92% of the culture. After washing out nocodazole and incubating 5 hr in α-factor, 93% of the culture was unbudded. Following the initial G2/M block small-budded cells were always <3% of the total cell population. (C) Establishment of repression following a G2/M block. A culture of YSH505 was blocked in G2/M, induced by addition of galactose for 8 hr, and then released from the G2/M block into galactose media and allowed to progress through the cell cycle (lanes 1, 3, 4, and 5). A parallel culture was blocked with nocodazole and released but never induced with galactose (lanes 2 and 6).