Abstract
Objectives. This case–control study investigated risk factors for campylobacteriosis in a rural population. Exposure to live farm animals was hypothesized to increase the risk for Campylobacter jejuni enteritis.
Methods. Incident cases from rural counties reported to the Michigan Department of Community Health and matched controls completed a self-administered postal questionnaire.
Results. Persons engaged in poultry husbandry had increased odds of campylobacteriosis (odds ratio = 6.884; 95% confidence interval (CI) = 1.438, 32.954). There was evidence for a dose–response relationship between the number of types of poultry contact and campylobacteriosis.
Conclusions. We estimate that 18% (95% CI = 6%, 30%) of Campylobacter cases occurring in rural populations are attributable to poultry husbandry. Cases occurred in individuals who were not poultry farmers by occupation.
Campylobacter jejuni is the most common cause of bacterial gastroenteritis in the United States.1 Including undiagnosed and unreported cases, it is estimated to affect over 2 million people every year. The annual cost of campylobacteriosis has been estimated at between $1.3 billion and $6.2 billion.2 This cost increases when sequelae, such as Guillain–Barré syndrome and reactive arthritis, are considered. Of additional concern, C jejuni and Campylobacter coli are becoming increasingly resistant to some antimicrobials.3
Risk factors for outbreak cases and sporadic cases differ. Outbreaks are typically due to raw milk4–6 or contaminated water consumption,7,8 but the vast majority of cases are sporadic. Identified risk factors for sporadic cases include consumption of undercooked chicken,9–11 contact with pets (especially puppies and kittens),12,13 and contact with diarrheic animals.12,14 Some ecological descriptive studies have shown that rates of infection are higher in rural areas than urban areas and that, among rural areas, farming regions have the highest rates.15 Living on or visiting a farm has been shown to increase the odds of infection,11 but a multicenter study in England and Wales showed decreased odds associated with occupational contact with livestock or their feces.12 A study of campylobacteriosis on rural Hopi and Navajo Indian reservations, showed an increased risk with ownership of farm animals.16
We conducted a study to determine the risk factors for C jejuni enteritis in rural communities. We hypothesized that exposure to food animals is a major risk factor and that the odds of infection change with exposure to different species.
METHODS
Design
A prospective matched case–control study design was implemented. The study duration was 1 year (October 2000–October 2001). Incident campylobacteriosis cases reported by physicians or clinical laboratories to the Michigan Department of Community Health were identified weekly. Michigan advocates that physicians report cases according to the nationally recognized case definition for confirmed or probable Campylobacter infections. A probable infection is defined as a clinically compatible case that is epidemiologically linked to a confirmed case, and a confirmed case is defined as one confirmed by a laboratory.17 Case subjects were contacted and invited to participate if they met the following inclusion criteria. First, they must have been residents of a rural county, defined as having a population of less than 70 000 in the 1990 census and not being adjacent to a major metropolitan center. Fifty-eight of Michigan’s 83 counties met this criterion. Second, to avoid potential problems with recall bias, the cases must have been reported to the Michigan Department of Community Health within 30 days of the onset of symptoms. Third, the cases must not have been part of an identified outbreak; fourth, they must have been the first in a household. Only first cases in a household were considered, because the effect of person-to-person transmission (although uncommon) was not of interest.
Because all cases were nonoutbreak and first in a household, and because Campylobacter enteritis is a laboratory diagnosis and not a clinical diagnosis, they were all considered laboratory confirmed as described above. It is possible that a few probable cases were included in the study. We do not have data to indicate which, if any, cases were probable. If these criteria were satisfied, case subjects were contacted by telephone about the study; if they expressed interest in participating, a postal questionnaire was sent.
Two control subjects were matched to each case subject by age group, sex, and county of residence (we chose these variables to eliminate them as potential confounders). Age groups were less than 1 year, 1 to 2 years, 3 to 4 years, 5 to 12 years, 13 to 19 years, 20 to 39 years, 40 to 59 years, and 60 years and older. The 2 control subjects had the same telephone area code and 3-digit prefix as the case subject. For selection, we used a sequential-digit dialing technique in which 1 digit was subtracted or added to the last 4 digits of a case subject’s phone number until 2 potential control subjects were identified. If a potential control subject was of the same age group and sex as the case subject and had not had any gastrointestinal symptoms during the 2 weeks before contact, he or she was sent a postal questionnaire.
Random-digit dialing was used because it enabled us to obtain a random sample of eligible control subjects in the same geographically defined area from which the case subjects came. Although we matched by county, most cases and control subjects were from the same town or adjacent towns. Identifying control subjects in this way may underrepresent those of low socioeconomic status because they often do not have telephone service; however, because only case subjects who could be contacted by telephone were invited to participate, case subjects and control subjects were probably comparable in that regard.
Questionnaire
The questionnaires sent to case and control subjects were identical except that case subjects were asked about behaviors 2 weeks before onset of symptoms and control subjects were asked about behaviors 2 weeks before contact by the investigators. After an informed consent form was signed, information was collected on demographic characteristics, foreign travel, antibiotic and antacid use, animal contact and husbandry behaviors, and food consumption habits. If the case or control subject was a child, the parent or guardian was asked to complete the questionnaire. Case or control subjects were considered to engage in animal husbandry if they participated in feeding, cleaning, or raising an animal for milk, eggs, or meat or had housed an animal in their home.
Data Analysis
Microsoft Access and Excel (Microsoft Corp, Redmond, Wash) were used to enter and organize data. Statistical analysis was performed with SAS version 8 (SAS Institute Inc, Cary, NC). A conditional logistic regression model was used for univariate and multivariate analyses. Independent variables associated with the outcome (P < .15) were then tested for association with a χ2 test. If 2 or more independent variables were significantly associated with each other (P < .05), the more biologically plausible variable was included in the model and the other was discarded. Variables that showed an association at the P < .15 level were then entered into a conditional logistic regression model for the multivariate analysis. We obtained the final model using hierarchical backward elimination of variables and applying statistical and epidemiological criteria for assessment of interaction and confounding. Interaction terms were considered significant if the P value for the term was less than .05 by the Wald test. Confounding was assessed by the impact of the potential confounder on the parameter estimate for the main effects (i.e., poultry husbandry). If removal of a confounding variable caused a change of 10% or more in the value of the parameter estimate, that variable was considered to be a confounder and was left in the model.
Modeling
The questionnaire asked about contact with particular species of animals, including domestic poultry, cattle, swine, horses, dogs, cats, and pet birds; each species question was followed by specific questions about the type of contact. These included questions about feeding, cleaning, or raising an animal for meat, eggs, or milk; housing any of these species in the home or garage; and having clothing contaminated with fecal material.
The results of the univariate analyses and odds for Campylobacter enteritis associated with each of these activities are shown in Table 1 ▶. When analyzed separately, these variables were highly associated with each other, as expected. Raising animals for eggs, milk, or meat almost always involves feeding, cleaning, and fecal contact. As a result, these variables were combined into 1 dichotomous summary variable, husbandry, for each species. Case and control subjects were considered to be positive for the husbandry exposure if they indicated exposure to any of the independent variables described above. This modeling of independent variables is biologically plausible because husbandry, or the care and raising of livestock, is a special kind of contact. It involves repeated, at least daily, direct contact with the animals, including contact with the fecal material of species that have the potential to carry Campylobacter organisms.
TABLE 1—
Univariate Analyses of Covariates Included in the Summary Husbandry Variables in a Case–Control Study of Sporadic Campylobacter jejuni Infections: Rural Michigan, 2000–2001.
| No. Exposed | No. Unexposed | |||||
| Case subjects | Control subjects | Case subjects | Control subjects | mOR | 95% CI | |
| Poultry housed in home or garage | 5 | 2 | 78 | 120 | 3.812 | 0.726, 20.014 |
| Poultry hatched on property | 4 | 2 | 79 | 120 | 5.767 | 0.621, 53.603 |
| Poultry raised for meat | 7 | 2 | 76 | 120 | 7.922 | 0.930, 67.472 |
| Poultry raised for eggs | 13 | 4 | 70 | 118 | 10.902 | 1.339, 88.744 |
| Feeding poultry | 9 | 8 | 74 | 114 | 2.169 | 0.619, 7.592 |
| Poultry-associated cleaning | 6 | 3 | 77 | 119 | 3.412 | 0.672, 17.339 |
| Clothing contaminated with poultry feces | 6 | 4 | 77 | 118 | 2.234 | 0.516, 9.678 |
| Calves born on property | 1 | 1 | 82 | 121 | 0 | 0 |
| Bovines housed in home or garage | 0 | 0 | 83 | 122 | 0 | 0 |
| Bovines kept for milk | 1 | 0 | 82 | 122 | 0 | 0 |
| Bovines kept for beef | 6 | 2 | 77 | 120 | 2.637 | 0.434, 16.013 |
| Feeding bovines | 6 | 3 | 77 | 119 | 2.189 | 0.513, 9.342 |
| Bovine-associated cleaning | 7 | 1 | 76 | 121 | 8.421 | 0.998, 71.041 |
| Clothing contaminated with bovine feces | 7 | 4 | 76 | 118 | 2.610 | 0.613, 11.119 |
| Pigs housed in home or garage | 0 | 0 | 83 | 122 | 0 | 0 |
| Pigs born on property | 3 | 0 | 80 | 122 | 0 | 0 |
| Pig kept as pet | 2 | 0 | 81 | 122 | 0 | 0 |
| Pigs raised for meat | 1 | 0 | 82 | 122 | 0 | 0 |
| Feeding pigs | 2 | 1 | 81 | 121 | 2.561 | 0.225, 29.120 |
| Pig-associated cleaning | 1 | 0 | 82 | 122 | 0 | 0 |
| Clothing contaminated with pig feces | 4 | 1 | 79 | 121 | 6.275 | 0.689, 57.165 |
Note. mOR = matched odds ratio; CI = confidence interval.
RESULTS
Of the 191 C jejuni enteritis cases reported to the Michigan Department of Community Health from rural counties during the year of our study, 50 were not eligible for inclusion. Forty-three cases were not eligible because their case report was received more than 30 days after symptom onset, and 5 were not eligible because they were the second case in a household. Two other cases were not eligible because the subjects were not Michigan residents during the 2 weeks before their illness onset. Of the 141 eligible case subjects, we did not have contact with 31 (15 had unlisted numbers, 3 had disconnected numbers, 10 did not answer their telephones after repeated attempts, and 3 had moved or were hospitalized). Of the 110 case subjects with whom we had contact, 6 refused and 21 initially agreed to participate in the study but later changed their minds, were not sure they could remember the period before their illness, or did not return their postal questionnaire. A total of 83 cases thus participated in the study. Using the method described by Slattery et al.,18 we calculated a cooperation rate of 75% (the percentage of people contacted who were interviewed).
We contacted all case and control subjects 2 weeks after the questionnaire was sent to ensure that it had been received and to answer any questions that had arisen. If the case or control subject could not be contacted by telephone, a second copy of the questionnaire was sent. Questionnaires were completed for 83 cases and 122 controls. The response rate for case subjects (percentage of those selected and eligible for study who were interviewed) was 59% (83/141). To calculate the response rate for control subjects, we added the numbers of those interviewed and those not eligible; this number was then divided by the total number of those who refused, those not eligible, and those not interviewed. We interviewed 122 control subjects and 1936 of those contacted were not eligible; only 336 refused to participate and 2117 households were not interviewed. Thus, the response rate for control subjects was 47% (2058/4389).
Univariate Analyses
Contact with any food-producing animal (bovines, swine, or poultry) was significantly associated with illness (odds ratio [OR] = 4.722; 95% confidence interval [CI] = 1.737, 12.833). Of factors considered significant in the univariate analyses (P < .15), contact with adult domestic poultry (OR = 3.216; 95% CI = 0.811, 12.763) and participation in the care and raising of poultry (OR = 8.454; 95% CI = 1.877, 38.081) increased the odds of illness (Table 2 ▶). The care and raising of cattle was also associated with illness (OR = 3.058; 95% CI = 0.907, 10.307), as was the care and raising of swine (OR = 7.358; 95% CI = 0.845, 64.079) and horses (OR = 3.380; 95% CI = 0.860, 13.294). Contact with foals also conferred increased odds for infection (OR = 6.275; 95% CI = 0.689, 57.165).
TABLE 2—
Matched Odds Ratios (mORs) for Animal Contact, Demographic Characteristics, and Food Consumption Habits for Campylobacter jejuni Enteritis Case Subjects: Rural Michigan, 2000–2001.
| No. Exposeda | No. Unexposed | |||||
| Exposure | Case subjects | Control subjects | Case subjects | Control subjects | MOR | 95% CI |
| Adult poultry | 9 | 4 | 74 | 118 | 3.216 | 0.811, 12.763 |
| Poultry husbandry | 18 | 8 | 65 | 114 | 8.454 | 1.877, 38.081 |
| Bovine husbandry | 12 | 5 | 71 | 117 | 3.058 | 0.907, 10.307 |
| Swine husbandry | 5 | 1 | 78 | 121 | 7.358 | 0.845, 64.079 |
| Equine husbandry | 9 | 5 | 74 | 117 | 3.380 | 0.860, 13.294 |
| Foals | 4 | 2 | 79 | 120 | 6.275 | 0.689, 57.165 |
| Farm | 21 | 12 | 62 | 110 | 2.484 | 1.041, 5.930 |
| Undercooked poultry | 6 | 26 | 77 | 96 | 0.180 | 0.052, 0.622 |
| Undercooked pork | 4 | 18 | 79 | 103 | 0.333 | 0.110, 1.013 |
Note. CI = confidence interval.
aExposures were significant in univariate analysis (P < .15).
We inquired about consumption and preparation of poultry, ground beef, and pork. Of these, only the consumption of undercooked pork and poultry was significant in the univariate analyses, but the effect of these undercooked meats was protective (OR = 0.333 and 0.180 for pork and poultry, respectively). Neither contact with adult cats (OR = 0.837; 95% CI = 0.453, 1.544) and kittens (OR = 0.546; 95% CI = 0.219, 1.364) nor consumption of raw milk (OR = 1.151; 95% CI = 0.408, 3.245) was associated with illness in our study. Eleven percent of control subjects and 10% of case subjects reported raw milk consumption in the 2 weeks before contact or illness, respectively.
Of the other exposures, including foreign travel, living on a farm, taking antibiotics or antacids, and having problems with rodents or houseflies in the home, only living on a farm was associated with illness (OR = 2.484; 95% CI = 1.041, 5.930) (Table 2 ▶).
We considered the interactions of antacid use, antibiotic use, and poultry exposure with the other animal contact and food consumption factors; none was significant.
Assessment of Associations Between Independent Variables
Equine husbandry and exposure to foals were strongly associated with poultry husbandry and bovine husbandry. Because chickens and cows are known to be important reservoirs of C jejuni, whereas horses are not,19 the horse exposure variables were dropped from the model. Similarly, farm exposure was very highly associated with animal husbandry, but specific animal exposure is more biologically plausible than general farm exposure. Farm exposure was, therefore, dropped from the model. Farm exposure was not as strongly associated with campylobacteriosis as were the animal husbandry variables.
Multivariate Model
Factors significant in the univariate model were entered into the multivariate model. These included poultry husbandry; cattle husbandry; swine husbandry; consuming poultry that was pink at the center, had red juices running from the meat, or was raw; and consuming pork that was pink at the center, had red juices running from the meat, or was raw. The consumption of undercooked poultry and pork was not statistically significant, nor was there evidence for confounding, so they were removed from the model. In the final model, only poultry husbandry (OR = 6.884; 95% CI = 1.438, 32.954) was associated with C jejuni enteritis. Swine and cattle husbandry showed increased risk, but their effect was not significant after poultry husbandry was controlled for (Table 3 ▶).
TABLE 3—
Final Multivariate Model Used in Case–Control Study of Sporadic Campylobacter jejuni Infections: Rural Michigan, 2000–2001.
| Exposure | mOR | 95% CI | P |
| Poultry husbandry | 6.884 | 1.438, 32.954 | .0158 |
| Bovine husbandry | 2.447 | 0.657, 9.114 | .1822 |
| Swine husbandry | 2.149 | 0.178, 25.995 | .5477 |
Note. mOR = matched odds ratio; CI = confidence interval.
Dose–Response Relationship
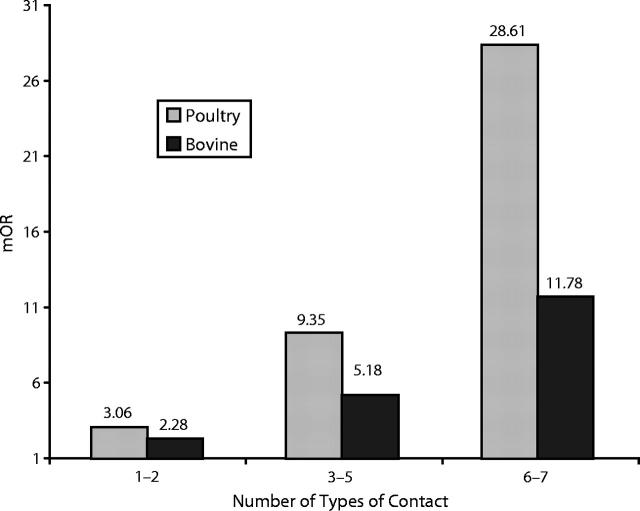
Because we found a strong association with poultry and bovine husbandry exposures, we investigated these variables to look for a dose–response relationship. From the 7 questions asked about husbandry exposure, 3 categories were created and modeled as an ordinal variable. The lowest husbandry category was 1 or 2 exposures, the middle category was 3 to 5 exposures, and the highest category was 6 or 7 exposures. A dose–response relationship was observed for poultry and bovine husbandry, but no cases had 6 or 7 of the exposure variables for these species. Because the variable was modeled as an ordinal one, the odds for the second and third category were calculated from the parameter estimate for the first category. The results are shown in Figure 1 ▶.
FIGURE 1—
Demonstration of a dose–response relationship between exposure to poultry and bovine husbandry and Campylobacter jejuni infection, by matched odds ratio (mOR).
Analysis of Nonresponders
The response rate for case subjects was 57%. We compared the age distribution, week of onset, and sex of responders and nonresponders. There was no significant difference between the 2 groups. Gastrointestinal illness case investigation reports were available for 17 of the 27 case subjects who refused to participate or did not respond. These reports are the results of a telephone interview between a public health nurse at the local health department and the case subject. Of the 17 reports, 2 were blank and 1 case subject had declined to give information, leaving 14 reports for analysis. Two of the 14 case subjects reported contact with food animals: 1 with poultry and 1 with sheep.
There was no significant difference between responders and nonresponders in contact with farm animals (Fisher exact test; P = .1280). Nonresponders were less likely to have reported contact with companion animals, however, than were responders (Fisher exact test; P = .0014). These findings suggest that there was not a response bias on food animals, but our study may not have had sufficient power to find such a bias if it did exist. The response rate for control subjects was 48%. There was no significant difference regarding age or sex between control subjects who responded and those who did not. Data were not collected on farm or companion animal exposure for control subjects who did not return their questionnaires.
DISCUSSION
We found that contact with farm animals was a significant risk factor for C jejuni enteritis in rural areas. Specifically, the care and raising of poultry increased the odds for disease by almost 7 times over and above the odds from husbandry of other species known to be reservoirs for Campylobacter organisms. Although other studies have documented the association between poultry contact and Campylobacter enteritis, this study is the first to characterize the contact as husbandry and to show that the more types of poultry husbandry one practices (feeding, cleaning, raising for meat, etc.), the greater the odds of contracting campylobacteriosis. Although we were not able to quantify the effects of specific husbandry exposures in a multivariate analysis owing to the high level of association between exposures, raising poultry for eggs conferred the highest odds for infection in univariate analysis (Table 1 ▶). Additionally, we found evidence of a dose–response relationship for increased odds of infection with increasing kinds of farm animal contact. Of the case subjects who reported engaging in poultry husbandry, only 2 had occupations related to farming; neither of these case subjects was a poultry farmer. In this population, those exposed were probably hobby farmers with small backyard flocks. We did not collect information on the use of antibiotics in feed or for treatment. We estimated that 18% (95% CI = 6%, 30%) of Campylobacter cases occurring in this population are attributable to poultry husbandry.20
Ecological studies have indicated that the incidence rate for C jejuni infection is higher in rural areas than in urban areas,21 especially in farming communities.15 Raw milk consumption,15,22 contact with farm animals,16 and daily contact with chickens or hens23 have been identified as potential risk factors in rural populations. This study confirms the findings of previous research implicating farm animal contact and further defines that contact as activities related to the care and rearing of poultry.
We did not find that raw milk consumption was a risk factor in our study. This finding may be a result of overmatching on location or because raw milk consumption was a more common practice among control subjects than was anticipated in power calculations. In a survey of milk producers, 35% reported drinking raw milk.24 Additionally, regular raw milk consumption has been shown to cause an elevated anti–C jejuni antibody titer that protects against symptomatic infection.25 Consumption of undercooked poultry or pork was also not a significant risk factor in this study. In a study of food consumption habits, rural residents were significantly less likely to eat undercooked hamburgers than were urban dwellers.26 It has been suggested that safe food preparation habits are learned through experience. If rural residents cook at home more frequently than their urban counterparts, undercooked meat may not be a major risk factor for them.
We did not find any association with exposure to cats or kittens, in contrast to other studies that have found such exposure to be significantly positively associated with illness.9,10 This may be explained by the fact that the previous studies took place in urban environments and that, although cats are fairly ubiquitous on farms, their presence is discouraged around the chicken coop and families may have little actual contact with them.
Owing to the relatively small number of case subjects enrolled, this study did not have sufficient power to find other significant associations; for example, the power to detect an association with bovine husbandry was calculated at only 23%.27 A study with a 2-year duration may enroll enough case subjects to find these associations. In addition, a postal questionnaire was used, a method that has the disadvantage of a lower response rate. The findings of this study, however, confirm previous investigations and provide a basis for additional research.
Case subjects who responded to the questionnaire were more likely to have association with companion animals than were those who did not respond. Although questions were asked about a variety of known risk factors for C jejuni, most of the questions involved animal contact. It is possible that case subjects who felt that the questionnaire did not apply to them (i.e., they had no animal contact) did not return it. We did not collect the data to measure this potential bias in control subjects. A large number of control subjects had contact with pets (88%); perhaps control subjects also were more motivated to return the questionnaire if they had contact with companion animals.
This study illustrates that, in rural areas, the care and raising of farm animals, particularly poultry, confers an increased risk for C jejuni enteritis. Understanding the risk factors for C jejuni enteritis is important because appropriate education and prevention efforts may help to decrease the incidence not only of the infection but also of its sequelae, including Guillain–Barré syndrome and reactive arthritis. Guillain–Barré syndrome is a subacute polyneuropathy affecting motor, sensory, and autonomic nerves that supply the limbs and respiratory muscles. Cranial nerves also may become involved. The mortality rate from Guillain–Barré syndrome is approximately 10%, and recovery is often incomplete, delayed, or both.28 The risk for Guillain–Barré syndrome is estimated to be about 100 times higher in persons with symptomatic C jejuni enteritis.29 Reactive arthritis may cause pain and incapacitation for several weeks to months in approximately 1% of C jejuni cases.30 To be most effective, public health measures aimed at prevention should be population specific.
Acknowledgments
Funding for this study was provided by the Population Medicine Center and the National Food Safety Center at Michigan State University.
Human Participant Protection This study was approved by the Michigan State University committee on research involving human subjects.
Contributors R. C. Potter conducted the study, helped develop the questionnaire, analyzed the data, and wrote the article. J. B. Kaneene planned the study, assisted with questionnaire development, supervised data collection and analysis, and contributed to writing the article. W. Hall helped plan the study, assisted with questionnaire development, and contributed to writing the article.
Peer Reviewed
References
- 1.Centers for Disease Control and Prevention. Preliminary FoodNet data on the incidence of foodborne illnesses—selected sites, United States, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:241–246. [PubMed] [Google Scholar]
- 2.Buzby JC, Allos BM, Roberts T. The economic burden of Campylobacter-associated Guillain-Barre syndrome. J Infect Dis. 1997;176(suppl 2):S192–S197. [DOI] [PubMed] [Google Scholar]
- 3.Engberg J, Aarestrup FM, Taylor DE, Gerner-Smidt P, Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg Infect Dis. 2001;7:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornblatt AN, Barrett T, Morris GK, Tosh FE. Epidemiologic and laboratory investigation of an outbreak of Campylobacter enteritis associated with raw milk. Am J Epidemiol. 1985;122:884–889. [DOI] [PubMed] [Google Scholar]
- 5.Harris NV, Kimball TJ, Bennett P, Johnson Y, Wakely D, Nolan CM. Campylobacter jejuni enteritis associated with raw goat’s milk. Am J Epidemiol. 1987;126:179–186. [DOI] [PubMed] [Google Scholar]
- 6.Evans MR, Roberts RJ, Ribeiro CD, Gardiner D, Kembrey D. A milk-borne Campylobacter outbreak following an educational farm visit. Epidemiol Infect. 1996;117:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St. Louis ME. Water-related disease outbreaks, 1985. MMWR CDC Surveill Summ. 1988;37(SS-2):15–24. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington County Fair—New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:803–805. [PubMed] [Google Scholar]
- 9.Hopkins RS, Olmsed R, Istre GR. Endemic Campylobacter jejuni infection in Colorado: identified risk factors. Am J Public Health. 1984;74:249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deming MS, Tauxe RV, Blake PA, et al. Campylobacter enteritis at a university: transmission from eating chicken and from cats. Am J Epidemiol. 1987;126:526–534. [DOI] [PubMed] [Google Scholar]
- 11.Friedman C, Reddy S, Samual M, et al. Risk factors for sporadic Campylobacter infections in the United States: a case–control study on FoodNet sites. Paper presented at: 2nd International Conference on Emerging Infectious Diseases; July 16–19, 2000; Atlanta, Ga.
- 12.Adak GK, Cowden JM, Nicholas S, Evans HS. The Public Health Laboratory Service national case–control study of primary indigenous sporadic cases of Campylobacter infection. Epidemiol Infect. 1995;115:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal KR, Slack RCB. Diabetes mellitus, antisecretory drugs and other risk factors for Campylobacter gastro-enteritis in adults: a case–control study. Epidemiol Infect. 1997;119:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed AM, Harris NV, DiGiacomo RF. The role of exposure to animals in the etiology of Campylobacter jejuni/coli enteritis. Am J Epidemiol. 1993;137:108–114. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JS, Cahoon FE, Hodge DS. Rate of Campylobacter spp. isolation in three regions of Ontario, Canada, from 1978 to 1985. J Clin Microbiol. 1986;24:876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engleberg NC, Correa-Villasenor A, North CQ, Crow T, Wells JG, Blake PA. Campylobacter enteritis on Hopi and Navajo Indian Reservations: clinical and epidemiologic features. West J Med. 1984;141:53–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep. 1997;46(RR-10):1–55. [PubMed] [Google Scholar]
- 18.Slattery ML, Edwards SL, Caan BJ, Kerber RA, Potter JD. Response rates among control subjects in case–control studies. Ann Epidemiol. 1995;5:245–249. [DOI] [PubMed] [Google Scholar]
- 19.Prescott JF, Bruin-Mosch CW. Carriage of Campylobacter jejuni in healthy and diarrheic animals. Am J Vet Res. 1981;42:164–165. [PubMed] [Google Scholar]
- 20.Kuritz SJ, Landis JR. Attributable risk estimation from matched case–control data. Biometrics. 1988;44:355–367. [PubMed] [Google Scholar]
- 21.Brieseman MA. A further study of the epidemiology of Campylobacter jejuni infections. N Z Med J. 1990;103:207–209. [PubMed] [Google Scholar]
- 22.Schmid GP, Schaefer RE, Plikaytis BD, et al. A one-year study of endemic campylobacteriosis in a midwestern city: association with consumption of raw milk. J Infect Dis. 1987;156:218–222. [DOI] [PubMed] [Google Scholar]
- 23.Studahl A, Andersson Y. Risk factors for indigenous Campylobacter infection: a Swedish case–control study. Epidemiol Infect. 2000;125:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrbach BW, Draughon FA, Davidson PM, Oliver SP. Prevalence of Listeria monocytogenes, Campylobacter jejuni, Yersinia enterocolitica, and Salmonella in bulk tank milk: risk factors and risk of human exposure. J Food Prot. 1992;55:93–97. [DOI] [PubMed] [Google Scholar]
- 25.Blaser MJ, Sazie E, Williams P. The influence of immunity on raw milk–associated Campylobacter infection. JAMA. 1987;257:43–46. [PubMed] [Google Scholar]
- 26.Altekruse SF, Yang S, Timbo BB, Angulo FJ. A multi-state survey of consumer food-handling and food-consumption practices. Am J Prev Med. 1999;16:216–221. [DOI] [PubMed] [Google Scholar]
- 27.Walter SD. Matched case–control studies with a variable number of controls per case. Appl Statistics. 1980;29:172–179. [Google Scholar]
- 28.Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997;176(suppl 2):S92–S98. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy N, Giesecke J. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. Am J Epidemiol. 2001;153:610–614. [DOI] [PubMed] [Google Scholar]
- 30.Skirrow MB, Blaser MJ. Clinical aspects of infection. In: Nachamkin I, Blaser MJ, eds. Campylobacter. 2nd ed. Washington, DC: American Society for Microbiology; 2000:79.