Figure 3.
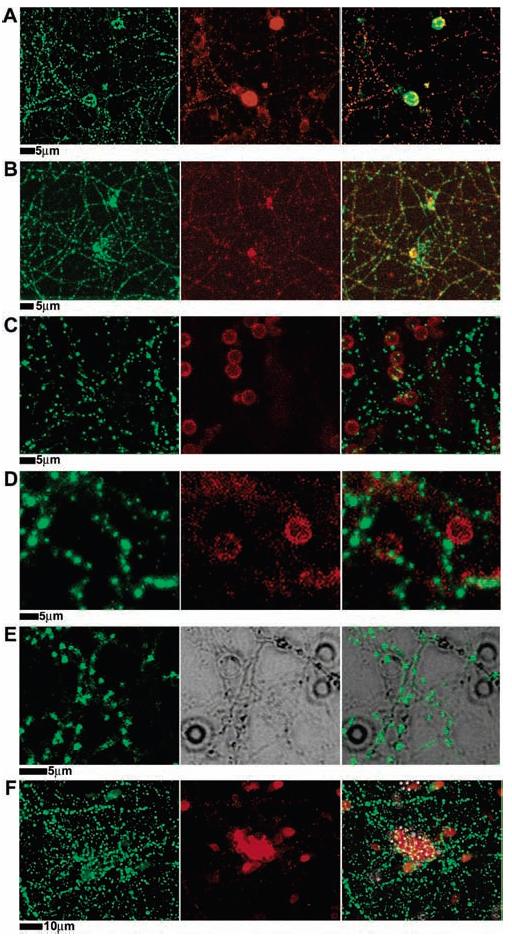
Presynaptic differentiation triggered by neuroligin-1 on the surface of cells or on lipid bilayer-coated membranes. (A) PC12 cells transfected with neuroligin-1–IRES–GFP (pseudo-colored red for consistency with other panels) were added to a 12 day old hippocampal culture, incubated overnight, then fixed and stained with antisynapsin (green in all panels) to mark presynaptic synaptic vesicles in the axons of the neuron. Synaptic vesicles accumulate, in puncta, at the site of contact with the PC12 cell. (B–F) Twelve day old hippocampal cultures interacting with beads coated with (B) synthetic lipid bilayers containing GPI-linked neuroligin-1 (neuroligin-1 stained in red), (C) synthetic lipid bilayers with no added protein (plain membranes in red), (D) synthetic lipid bilayers containing GPI-linked alkaline phosphatase (GPI-linked alkaline phosphatase in red), (E) neither synthetic lipid bilayer nor protein, bare silica beads (middle panel is DIC image of beads and neurons), and (F) with chemically coupled neuroligin-1 on polystyrene beads (neuroligin-1 in red). Cultures were fixed 24 h following introduction of beads and were stained with anti-HA antibodies for either neuroligin-1 (red), GPI-linked alkaline phosphatase (red), or synapsin (green). Microbeads coated only with synthetic lipid bilayers included 1% Texas red–DPPE in the bilayer for fluorescence imaging. Only beads with synthetic lipid bilayers containing GPI-linked neuroligin-1 induced presynaptic differentiation of the neurons, as indicated by synapsin accumulation in axons contacting the beads.
