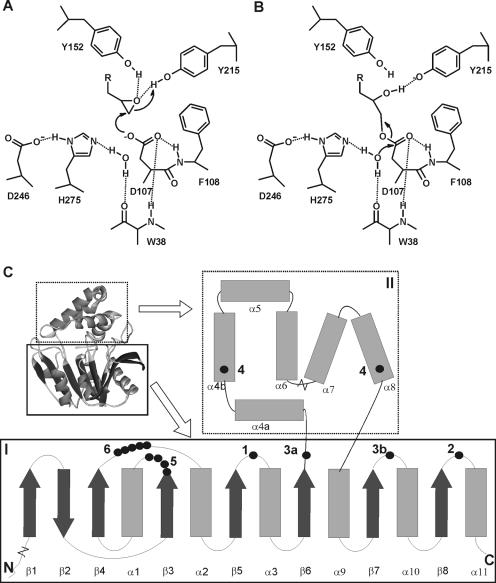
FIG. 1.
Structure and mechanism of α/β-hydrolase fold epoxide hydrolases. The active site shown in panels A and B is from the epoxide hydrolase from A. radiobacter AD1 (EchA) (31, 35, 36). (A) Tyrosine-assisted ring opening of the epoxide by the nucleophilic aspartate. (B) Hydrolysis of the alkyl-enzyme intermediate by an activated water molecule. (C) General topology and structure of α/β-hydrolase fold epoxide hydrolases. I, main domain with α/β-hydrolase fold; II, cap domain; 1, catalytic nucleophile (D107 in EchA); 2, histidine base (H275); 3, charge relay acid, which can be positioned at either 3a or 3b (D246 is at position 3b); 4, ring-opening tyrosines (Y152/Y215); 5, H-G-X-P motif (the X is usually an aromatic residue in epoxide hydrolases [W38]); 6, G-X-Sm-X-S/T motif. The region of helix α4a can be nonhelical or missing in some epoxide hydrolases. The length of the loop between helixes α6 and α7 can vary considerably. Extensions with additional domains of 40 to 300 amino acids can occur at both the N and C termini.