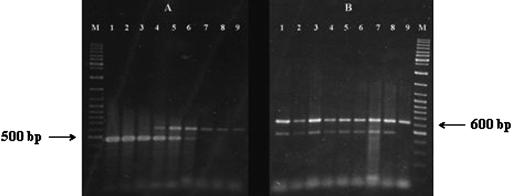
FIG. 6.
Detection of E. sakazakii in reconstituted infant formula by PCR in the presence of S. enterica serovar Typhimurium. Primer pair ESSF and ESSR and the template preparation, amplification conditions, and gel electrophoresis method described in Materials and Methods were employed. (A) PCR amplifications carried out on infant formula samples containing 108 CFU/ml serovar Typhimurium along with 108 to 101 CFU/ml of E. sakazakii. Lanes: M, molecular weight marker (GeneRuler DNA Ladder Mix; Fermentas, Hanover, Md.); 1 to 8, PCR products amplified from infant formula containing 108 to 101 CFU/ml of E. sakazakii (corresponding samples on each of the lanes also contained 108 CFU/ml serovar Typhimurium); 9, control PCR run with infant formula containing 108 CFU/ml serovar Typhimurium alone. (B) PCR amplifications carried out on infant formula samples containing 103 CFU/ml E. sakazakii along with 108 to 101 CFU/ml of serovar Typhimurium. Lanes: M, molecular weight marker (GeneRuler DNA Ladder Mix; Fermentas, Hanover, Md.); 1 to 8, PCR products amplified from infant formula containing 108 to 101 CFU/ml of serovar Typhimurium (corresponding samples on each of the lanes also contained 103 CFU/ml E. sakazakii); 9, control PCR run with infant formula containing 108 CFU/ml serovar Typhimurium alone.