Abstract
Objectives. We sought to determine hepatitis B virus (HBV) infection prevalence, associated exposures, and incidence among male inmates at a state correctional facility.
Methods. A cross-sectional serological survey was conducted in June 2000, and susceptible inmates were retested in June 2001.
Results. At baseline, 230 inmates (20.5%; 95% confidence interval [CI]=18.2%, 22.9%) exhibited evidence of HBV infection, including 11 acute and 11 chronic infections. Inmates with HBV infection were more likely than susceptible inmates to have injected drugs (38.8% vs 18.0%; adjusted prevalence odds ratio [OR]=3.0; 95% CI=1.9, 4.9), to have had more than 25 female sex partners (27.7% vs 17.5%; adjusted prevalence OR=2.0; 95% CI=1.4, 3.0), and to have been incarcerated for more than 14 years (38.4% vs 17.6%; adjusted prevalence OR=1.7; 95% CI=1.1, 2.6). One year later, 18 (3.6%) showed evidence of new HBV infection. Among 19 individuals with infections, molecular analysis identified 2 clusters involving 10 inmates, each with a unique HBV sequence.
Conclusions. We documented ongoing HBV transmission at a state correctional facility. Similar transmission may occur at other US correctional facilities and could be prevented by vaccination of inmates.
In comparison with the general population, individuals incarcerated in correctional systems bear a disproportionately greater burden of infectious diseases, including hepatitis B virus (HBV) infection. The prevalence of past HBV infection among incarcerated individuals has been reported to range from 8% to 43%, as compared with 4.9% in the population of the United States as a whole.1–6 This higher rate among inmates reflects the overrepresentation in this population of risk factors, such as injection drug use and unprotected sex with multiple partners, associated with HBV infection.1–5 Since 1982, the Advisory Committee on Immunization Practices has recommended hepatitis B vaccination among inmates at long-term correctional facilities who have a history of high-risk behaviors.7 Although vaccination is offered to some inmate populations in several state and federal correctional settings, universal immunization of incarcerated individuals is not common.8
The risk behaviors that can transmit HBV infection are often prohibited at correctional facilities; however, the rate at which such behaviors nonetheless occur has been poorly quantified.8–12 According to the limited information available, the incidence of HBV infection among incarcerated individuals in the United States is estimated to range from 0.8% to 1.4% per year.13,14 In addition, as a result of security, sentencing, and appeals processes, inmates move frequently among different correctional facilities, providing the opportunity for a large, mobile population to come in contact with a reservoir of others with chronic HBV infection.13,14
In June 2000, after a long-term inmate at a state correctional facility in Georgia had been found to have an acute HBV infection, we conducted a serological survey designed to identify additional cases and sources of HBV infection and offered vaccination to susceptible inmates housed in the dormitory of the index patient and cellmates of chronically infected inmates.15 Fifty-seven inmates agreed to be vaccinated.
In June 2001, during a facility-wide hepatitis B vaccination campaign implemented in response to another case of acute hepatitis B identified at the same correctional facility, we conducted a second serological survey among inmates who had been identified as susceptible to HBV infection during the first survey and had not been subsequently vaccinated. The objectives of this second survey were to identify unrecognized cases and to determine the correctional facility’s annual incidence of HBV infection.
METHODS
Population
The high-security correctional facility at which the study was conducted accommodates up to 1340 male inmates, approximately a third of whom are transferred or released each year. Inmates are housed in one of 14 dormitories; each dormitory holds up to 96 inmates. All inmates incarcerated at the correctional facility in June 2000 were eligible to participate in the baseline serological survey. To measure the incidence of HBV infection, we conducted a follow-up survey in June 2001 by collecting a second serological specimen from inmates who were found to be susceptible to HBV infection during the June 2000 baseline survey and had not received hepatitis B immunization in the intervening year. We also collected specimens from inmates who were new to the facility or had chosen not to participate in the baseline serological survey. Acute or chronic infections identified from among these specimens were included in a genetic relatedness analysis.
Data Collection
Inmates were provided information about HBV infection, its modes of transmission, the risks and benefits of vaccination, and the purpose of the investigation. After providing written informed consent, inmates who chose to participate submitted a blood sample for serological testing. During the June 2000 baseline serological survey, participating inmates were also asked to complete a questionnaire gathering demographic and exposure information, including age, race/ethnicity, and known and potential risk factors for HBV infection (e.g., using injection drugs, engaging in high-risk sexual activity, having a tattoo applied) both before and during imprisonment. Inmates completed these self-administered surveys in a private area within the facility medical unit. Staff from the Centers for Disease Control and Prevention (CDC) and the Georgia Department of Human Resources supervised the data collection. In addition, all inmates who showed evidence of new HBV infection in June 2001 were interviewed to assess potential exposures to HBV during the intervening year.
Serological Testing
Serum specimens were tested at the CDC Hepatitis Reference Laboratory to assess total and IgM antibodies to hepatitis B core antigen (anti-HBc), antibodies to hepatitis B surface antigen (anti-HBs), and hepatitis B surface antigen (HBsAg); standard assays were used (CORZYME, CORZYME-M, AUZYME monoclonal, HBsAg Quantitation Panel; Abbott Laboratories, Abbott Park, Ill). Participants were grouped into one of the following categories: acute HBV infection (positive for total and IgM anti-HBc with or without HBsAg), chronic infection (positive for HBsAg and total anti-HBc but negative for IgM anti-HBc), or resolved infection (positive for total anti-HBc but negative for IgM anti-HBc and HBsAg). Participants were presumed to be susceptible to infection if they had negative results for both total anti-HBc and HBsAg.
Specimens collected in June 2001 were first tested for total anti-HBc. Positive specimens were tested for IgM anti-HBc, anti-HBs, and HBsAg. Inmates who were negative for all hepatitis B markers (total anti-HBc, HBsAg, IgM anti-HBc, and anti-HBs) in 2000 and positive for total anti-HBc and HBsAg, IgM anti-HBc, or anti-HBs in 2001 were classified as having seroconverted.
Nucleic Acid Sequencing of HBV DNA
Among inmates with serological evidence of acute infection, chronic infection, or seroconversion, a modification of the package insert protocol for the MasterPure Complete DNA and RNA Purification Kit (Epicentre Technologies; Madison, Wis) was used in extracting HBV DNA from 50 μL of serum. A 250-base-pair segment of the S region (encoding for the surface antigen) of the HBV genome was amplified by nested polymerase chain reaction (PCR), as described previously.16,17
PCR products were purified (QIAquick spin columns; Qiagen Inc, Valencia, Calif), and automated sequencing was performed (ABI model 373 or 377; Applied Biosystems, Foster City, Calif), as described previously.17 Sequencing reactions were done with a prism dye or dRhodamine terminator cycle sequencing kit according to the manufacturer’s protocol. Sequence data were further analyzed via Sequence Navigator (Applied Biosystems, Foster City, Calif) and GCG Wisconsin Package (Accelrys Inc; San Diego, Calif) software.18 Neighbor-joining methodology was used to construct a phylogenetic tree.
Statistical Analyses
Prevalence of HBV infection, inmate demographic and exposure characteristics, and incidence of HBV infection were estimated via sample proportions; exact binomial computation was used in estimating 95% confidence intervals (CIs). After adjustment for age, an analysis of the relationship between HBV infection and inmate characteristics was conducted. A multivariate logistic regression model was also developed to determine associations between HBV infection and reported demographic characteristics, known risk factors, and other exposures. All variables were initially included in the model. Possible interactions between age or length of incarceration and other variables were explored. Only statistically significant demographic and exposure characteristics were retained in the final multivariate logistic model. Significance values below the .05 level were considered significant. Stata statistical software (Stata Corp, College Station, Tex) was used in conducting all statistical calculations.
RESULTS
Description of Participants
Of the 1351 inmates housed at the correctional facility in June 2000, 1124 (83%) participated in the investigation. The median age of participating inmates was 32.5 years (range: 18–71 years), the median duration of formal education was 11 years (range: 0–18 years), and the median duration of incarceration was 7 years (range: 0–40 years). The majority (66%) of participants were African American; 7% were Hispanic. Reported lifetime risk factors included history of a sexually transmitted disease (STD) (38%), history of injection drug use (12%), history of sex with a male partner (8%), and more than 25 lifetime female sex partners (36%). Reported risk factors during incarceration included having a tattoo applied (48%), engaging in sex with a male partner (5%), and using injection drugs (3%). Data on nonparticipating inmates were not available.
Baseline Serological Survey of Inmates: June 2000
Laboratory results identified 11 (1.0%) participants with acute infections, 11 (1.0%) with chronic infections, and 208 (18.5%) with resolved infections, yielding a total of 230 participants (20.5%; 95% CI=18.1%, 22.9%) with HBV infections at baseline (Table 1 ▶). Except for one symptomatic inmate with acute HBV infection, neither inmates with acute infections nor those with chronic infections were aware of their infection status. The prevalence of HBV infection among participants who had been incarcerated more than 14 years was 38.4% (95% CI=30.9%, 46.3%), as compared with 15.1% (95% CI=12.4%, 18.2%) of those who had been incarcerated for 7 or fewer years. Among participants older than 35 years, 30.8% (95% CI=26.5%, 35.4%) had evidence of HBV infection, in comparison with 10.8% (95% CI=7.2%, 15.3%) of those 25 years or younger.
TABLE 1—
Prevalence of Hepatitis B Virus (HBV) Infection Among Inmates, by Selected Demographic and Behavioral Characteristics: June 2000 (n = 1122)
| No. of Inmates | No. of Inmates With HBV Infection | Inmates With HBV Infection, % (95% CI) | |
| Age, y | |||
| 25 or below | 250 | 27 | 10.8 (7.2, 15.3) |
| 26–35 | 427 | 66 | 15.5 (12.2, 19.2) |
| More than 35 | 435 | 134 | 30.8 (26.5, 35.4) |
| Race/ethnicity | |||
| White | 269 | 49 | 18.2 (13.8, 23.4) |
| African American | 735 | 153 | 20.8 (17.9, 23.9) |
| Hispanic | 74 | 18 | 24.3 (15.1, 35.7) |
| Other | 31 | 7 | 22.6 (9.6, 41.1) |
| Less than 12 years of education | |||
| Yes | 715 | 144 | 20.1 (17.3, 23.3) |
| No | 369 | 81 | 22.0 (17.8, 26.5) |
| Total no. of years incarcerated | |||
| 7 or fewer | 634 | 96 | 15.1 (12.4, 18.2) |
| 8–14 | 311 | 70 | 22.5 (18.0, 27.6) |
| More than 14 | 164 | 63 | 38.4 (30.9, 46.3) |
| History of sexually transmitted disease | |||
| Yes | 423 | 102 | 24.1 (20.1, 28.5) |
| No | 684 | 124 | 18.1 (15.3, 21.2) |
| History of injection drug use | |||
| Yes | 129 | 50 | 38.8 (30.3, 47.7) |
| No | 982 | 177 | 18.0 (15.7, 20.6) |
| History of sex with a male partner | |||
| Yes | 62 | 20 | 32.3 (20.9, 45.3) |
| No | 1050 | 207 | 19.7 (17.3, 22.2) |
| Lifetime no. of female sex partners | |||
| Fewer than 10 | 253 | 55 | 21.7 (16.8, 27.3) |
| 10–25 | 416 | 62 | 14.9 (11.6, 18.7) |
| More than 25 | 375 | 104 | 27.7 (23.3, 32.6) |
| Application of tattoo (lifetime) | |||
| Yes | 683 | 139 | 20.4 (17.4, 23.6) |
| No | 379 | 76 | 20.0 (16.1, 24.4) |
| Injection drug use during incarceration | |||
| Yes | 33 | 12 | 36.4 (20.4, 54.9) |
| No | 1083 | 217 | 20.0 (17.7, 22.5) |
| Sex with male partner during incarceration | |||
| Yes | 51 | 16 | 31.4 (19.1, 45.9) |
| No | 1061 | 212 | 20.0 (17.6, 22.5) |
| Application of tattoo during incarceration | |||
| Yes | 532 | 112 | 21.1 (17.7, 24.8) |
| No | 580 | 117 | 20.2 (17.0, 23.7) |
| Total | 1122a | 230 | 20.5 (18.2, 23.0) |
Note. CI = confidence interval. Data were Incomplete for some characteristics.
aHBV infection was determined by the presence of antibody to hepatitis B core antigen.
In the age-adjusted univariate analysis, non-White race/ethnicity, history of injection drug use, more than 25 lifetime female sex partners, history of an STD, incarceration for more than 14 years, and having a tattoo applied while incarcerated were significantly associated with HBV infection (Table 2 ▶). History of injection drug use exhibited the strongest association. Risk behaviors not significantly associated with HBV infection included history of injection drug use while incarcerated (adjusted prevalence odds ratio [OR]=1.82; 95% CI=0.86, 3.81), history of sex with a male partner (adjusted prevalence OR = 1.66; 95% CI = 0.93, 2.95), history of sex with a male partner while incarcerated (adjusted prevalence OR=1.71; 95% CI=0.91, 3.23), and having a tattoo applied (adjusted prevalence OR=1.37; 95% CI= 0.98, 1.91).
TABLE 2—
Risk Factors for Hepatitis B Virus Infection Among Inmates, June 2000: Age-Adjusted and Multivariate Logistic Regression Analysis Results (n = 1122)
| Age-Adjusted Prevalence OR (95% CI) | Multivariate Logistica Prevalence OR (95%) | |
| Age, y | . . . | 1.05 (1.03, 1.07) |
| Race/ethnicity | ||
| White | Referent | Referent |
| African American | 1.60 (1.09, 2.34) | 2.63 (1.68, 4.13) |
| Hispanic | 2.19 (1.15, 4.20) | 3.82 (1.88, 7.77) |
| Other | 2.37 (0.93, 6.04) | 3.83 (1.46, 10.1) |
| History of injection drug use | 2.26 (1.51, 3.38) | 3.00 (1.85, 4.88) |
| History of sexually transmitted disease | 1.38 (1.02, 1.87) | . . . |
| Lifetime no. of female sex partners | ||
| Fewer than 10 | 1.16 (0.81, 1.66) | 1.97 (1.27, 3.04) |
| 10–25 | Referent | Referent |
| More than 25 | 1.64 (1.20, 2.24) | 2.03 (1.40, 2.96) |
| Incarcerated more than 14 years | 1.81 (1.22, 2.71) | 1.68 (1.08, 2.59) |
| Application of tattoo during incarceration | 1.44 (1.06, 1.98) | 1.46 (1.04, 2.06) |
Note. OR = odds ratio; CI = confidence interval.
aAll variables were initially included in the model, but only variables significant at the P < .05 level were retained in the final model; likelihood ratio χ29= 106, P < .0001.
The final multivariate logistic model retained the following as significant exposure variables: age, race/ethnicity, history of injection drug use, more than 25 lifetime female sex partners, fewer than 10 lifetime female sex partners, incarceration for more than 14 years, and having a tattoo applied while incarcerated (Table 2 ▶). Race/ethnicity and history of injection drug use were most strongly associated with HBV infection.
Follow-Up Serological Survey of Susceptible Inmates: June 2001
As of June 2001, 653 (73%) of the 894 susceptible inmates identified in June 2000 remained at the correctional facility, had not received any hepatitis B vaccine, and were invited to participate in the follow-up serological survey. Of these inmates, 503 (77%) consented to repeat testing. The median age of these participating inmates was 33 years (range: 19–72 years), and the median duration of incarceration was 8.5 years (range: 1–41 years); 322 (64%) were African American and 31 (6%) were Hispanic. The 150 susceptible inmates who chose not to participate were similar to participating inmates with respect to demographic characteristics and reported exposures at baseline (data not shown). Participating inmates were more likely to accept hepatitis B vaccine during the June 2001 facility-wide vaccination program than those not participating (87% vs 21%; P<.001). The overall vaccination acceptance rate was 68%.
A total of 18 (3.6%) inmates participating in the June 2001 survey had serological evidence of HBV infection during the intervening year, yielding an annual incidence of HBV infection of 3579 per 100 000. These 18 seroconverting inmates included 3 with acute infections, 1 with a chronic infection, and 14 with resolved infections. Their median age was 34 years (range: 22–48 years), and their median duration of incarceration was 7 years (range: 1–19 years); 16 (89%) were African American and 2 (11%) were White. Exposure information was subsequently collected on 15 (84%) of these inmates. Two inmates reported having had a tattoo applied during the past year, one of whom also reported having sex with a male partner during the previous year. None reported injection drug use during the past year, and only one reported clinical symptoms suggestive of acute hepatitis B.
Serological Testing of Other Inmates: June 2001
In the June 2001 survey, an additional 554 inmates not eligible to participate in the follow-up serological survey were present at the facility, including 132 who declined participation in the June 2000 baseline serological survey but were still housed at the facility and 423 who were new to the facility since June 2000. We obtained serological specimens from 366 (66%) of these inmates. Their median age was 32 years (range: 19–58 years), and 235 (64%) were African American. We identified 2 acute and 4 chronic cases. Both patients with acute infections had arrived at the facility since June 2000. Among the 188 nonparticipants, the median age was 32 years (range: 19–65 years); 155 (82%) were African American.
HBV Genetic Relatedness
Overall, 16 inmates with chronic infections, 16 with acute infections, and 13 who had seroconverted were identified from the 2 serological surveys and the specimens collected from inmates who were housed at the facility in June 2001 but were not eligible to participate in the follow-up serological survey. HBV DNA was detected in the serum samples of 14 inmates with chronic infections, 5 inmates with acute infections, and none of the inmates who had seroconverted.
Eleven unique nucleotide sequence patterns belonging to 3 HBV genotypes were identified (Figure 1 ▶). Eight (1F, 1G, 1I, 1J, 1M, 2O, 2P, 2R) of the 14 chronically infected inmates had unique HBV strains; these inmates had been incarcerated an average of 13.5 years (range: 3–25 years) and had been housed at the study correctional facility for an average of 2.5 years (range: 4 months–8 years). Reported risk factors for HBV infection among this group were history of an STD (38%), history of injection drug use (25%), having a tattoo applied during incarceration (38%), and more than 25 lifetime female sex partners (88%). One inmate (1H) with an acute HBV infection identified during the June 2000 serological survey also had a unique strain. This inmate had been incarcerated for 15 months, 5 of which were spent at the study facility; he denied any sexual contact, injection drug use, or tattooing during the previous 6 months.
FIGURE 1—
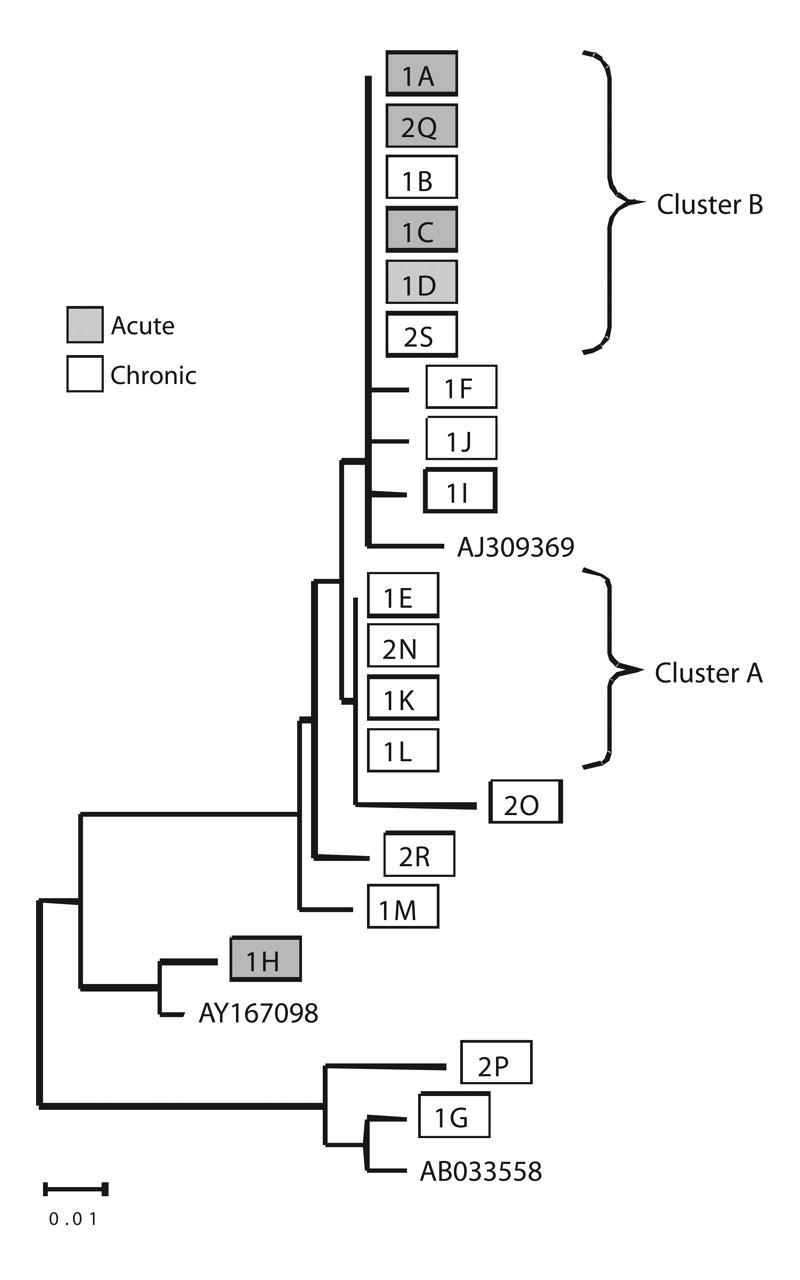
Genetic relatedness of hepatitis B virus (HBV) DNA from infected inmates: phylogenetic distribution derived from 145-base-pair segment from the surface antigen region.
Note. Samples designated with prefix “1” were collected during the baseline serological survey in June 2000; those with prefix “2” were collected during June 2001. The horizontal line at bottom left represents 1% nucleotide substitution for that horizontal branch length. HBV genotype reference strains were obtained from GenBank (accession numbers AJ309369 for genotype A, AY167098 for genotype B, and AB033558 for genotype D).
The 10 remaining sequences represented 2 strains. One strain (Cluster A in Figure 1 ▶) included sequences from 3 inmates (1L, 1E, 1K) with chronic infections identified during the baseline serological survey in June 2000 and a fourth inmate (2N) present at the correctional facility in June 2000 but not tested until June 2001. The chronically infected inmates with this strain had been incarcerated an average of 6.5 years (range: 3–11 years) and had been housed at the correctional facility for an average of 2.9 years (range: 2 months–6 years). Reported risk factors for HBV infection among this group included history of an STD (75%), having a tattoo applied during incarceration (50%), and more than 25 lifetime female sex partners (50%).
The second strain (Cluster B in Figure 1 ▶) included sequences from 2 inmates (1B, 2S) with chronic infections and 4 inmates with acute infections; among the latter, 3 (1A, 1C, 1D) were identified during the June 2000 baseline serological survey and 1 (2Q) during the June 2001 follow-up serological survey. These inmates had been incarcerated for a median of 7.8 years (range: 2.3–15 years) and had been housed at the facility for 2.6 years (range: 1.8–3.5 years). One chronic infection (2S) was newly acquired, as the inmate in question had been negative for all HBV markers during the June 2000 serological survey. Two inmates (1C, 2Q) with acute infections who exhibited this HBV strain reported having had sex with an inmate (1B) chronically infected with the same strain. Of the 3 remaining inmates, 2 infected with this strain reported having a tattoo applied during incarceration as their only potential exposure.
DISCUSSION
This investigation conducted at a state correctional facility documented both a high prevalence of HBV infection and a high rate of ongoing HBV transmission within the facility. Only one of the 11 inmates with an acute HBV infection identified in 2000 and one of the 18 inmates with an incident HBV infection identified in 2001 had clinical symptoms of acute hepatitis B; the remainder were identified through serological testing.
The annual incidence of HBV infection in this facility (3579 per 100000) was more than 120 times the estimated national incidence of HBV infection (29 per 100000) in 2000.19,20 If the incidence in this single facility were applied to the 43000 men currently incarcerated in the Georgia state correctional system, an estimated 1500 individuals would be infected during their incarceration.21 While the extent to which these findings can be generalized is unknown, a similar incidence was found in the Rhode Island correctional system.22 Furthermore, HBV transmission has been documented at other correctional facilities in Georgia.23 Because most inmates with acute or chronic infections in our investigation were not aware of their infection status, interventions designed to prevent transmission to close contacts could not have been implemented. In addition, an estimated one third of inmates in the Georgia state correctional system are released annually to the community or transferred to another correctional facility, resulting in these facilities serving as a reservoir for ongoing transmission of HBV.
No predominant mode of transmission was identified for incident cases of HBV infection. Sex with a male partner was associated with only one third of acute infections in the June 2000 outbreak investigation,15 and this risk factor was reported by only one of the 18 inmates identified 1 year later. Because sexual contact between inmates is prohibited in correctional facilities, such exposures were probably underreported in both the initial and follow-up investigations. Underreporting also might explain why these exposures were not strongly associated with HBV infection in the prevalence survey. One could speculate that in the case of some of the inmates, reported history of fewer than 10 lifetime female sex partners (which was associated with HBV infection in the multivariate logistic model) might have served as a proxy for sex with a male partner.
We identified additional inmate exposures that could have facilitated HBV transmission. Although illegal, injection drug use during incarceration was reported by a small proportion of the inmates. In addition, almost half of the inmates reported that they had had a tattoo applied while they were incarcerated. Tattooing instruments and injection equipment, if shared between individuals with HBV infection and individuals susceptible to infection, could potentially serve as transmission vehicles, particularly given the substantial number of inmates with chronic infections.
The clustering of unique HBV nucleotide sequences among inmates provides strong evidence of chains of transmission between these individuals. The inmates with chronic infections in Cluster A may have acquired these infections many years previously, either at the study facility or at another facility where this strain was circulating. However, the 6 inmates with the unique sequence in Cluster B were likely to have acquired their infection from each other, most likely at the study facility. While 2 inmates with acute infections had engaged in the risk behavior of sex with a male partner, the others may have acquired their infections through unreported risk factors or through nonsexual, inapparent transmission of the type observed in households.24,25 Similarly, the single inmate with an acute HBV infection whose nucleotide sequence pattern did not match those of other infected inmates may have acquired his infection from someone who transferred out of the facility before the June 2000 survey or chose not to participate in the study.
It is well documented that HBV infection can be transmitted to nonsexual household contacts of individuals with chronic infections through inapparent percutaneous exposures to blood.24,25 The potential for such transmission exists in prison settings, where inmates live in close proximity to one another and overcrowding is often present.7,26 In addition, events such as altercations may have the potential to result in exposures to HBV-contaminated body fluids.
This epidemiological investigation was initiated by a single case of acute hepatitis B and identified substantial ongoing chains of HBV transmission within a correctional facility. Acute HBV infection in adults is clinically evident less than half of the time.7 Although acute hepatitis B is a reportable condition in all states, it is rarely reported by correctional facilities. Many states, including Georgia, also require reporting of laboratory tests indicative of acute HBV infection. However, as occurred in Georgia, case reviews would be required to ascertain that the infection involved an individual housed at a correctional facility. The experience from this outbreak strongly suggests that single cases of acute hepatitis B identified in correctional facilities should be investigated to determine extent of transmission and to educate correctional and medical staff about prevention of HBV infection and management of infected individuals.26
Blood-borne infections other than HBV could be transmitted via the same mechanisms. If inmates with Human Immunodeficiency Virus (HIV) or hepatitis C virus (HCV) infections share injection or tattoo equipment, transmission of these infections could occur. In addition, despite correctional system prohibitions against sexual contact, sexual transmission of HIV infections and other STDs may occur.27 Education on behaviors that can transmit HBV and other infections should be provided to inmates, at time of entry as well as prior to release, as part of correctional facilities’ health education and risk reduction programs.8 Removing barriers to effective prevention, such as condom bans, could help prevent the spread of infection among inmates. Condoms have been available to inmates in the prison systems of Canada and other countries for many years.28
As demonstrated in this investigation, sustained control and prevention of HBV transmission within correctional systems is unlikely to occur without widespread vaccination of inmates because of the high prevalence of inmates with asymptomatic acute and chronic infections, the high prevalence among inmates of risk factors for infection, and the frequent transfer of inmates between correctional facilities. Failure to offer immunization to susceptible inmates throughout the study facility after the June 2000 serosurvey represented a missed opportunity. Studies of community-based cases of acute hepatitis B indicate that, each year, at least one third occur among individuals who report a history of incarceration, suggesting that vaccination of incarcerated individuals also has the potential to prevent infections in the community.29,30
Hepatitis B vaccination has been recommended for incarcerated individuals7,8 and has been shown to be cost effective from a societal perspective.31 Furthermore, it has been successfully delivered to individuals in correctional settings.32,33 Integrating immunization services to prevent HBV infection among incarcerated individuals and their community contacts, including offering hepatitis B vaccination to susceptible inmates, would go a long way toward eliminating HBV transmission in the United States.
Acknowledgments
We wish to acknowledge the following individuals for their assistance in this investigation: Dr R. Luke Shouse, Morehouse College of Medicine, and Dr Andrew Kroger, Emory University, Atlanta, Ga; Ruth Neeman, RN, Georgia Division of Public Health; William Kissel and the health care providers in the Georgia Department of Corrections who participated in the investigation; Dr Wendi Kuhnert, Dr Mike Purdy, and Tracy Greene, Hepatitis Reference Laboratory, Centers for Disease Control and Prevention; Dr Stephanie Bialek, Marcia Brooks, and Cindi Pecoraro (data collection); Mary Beth Weber and Darigg Brown (data entry); Richard Conlon and Drs Rob Lyerla, Annemarie Wasley, and Ian Williams of the Division of Viral Hepatitis, National Center for Infectious Diseases, Centers for Disease Control and Prevention (technical and administrative support); and Richard Kahn, National Center HIV, STD, and TB, Centers for Disease Control and Prevention (data collection).
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
Contributors A. J. Khan conducted the study, synthesized analyses, and led the writing. E. P. Simard assisted with the study and analyses. W. A. Bower assisted with the molecular analyses and interpretation. H. L. Wurtzel and K. D. Wagner assisted with the study. M. Khristova and O. V. Nainan assisted with the molecular analyses. K.E. Arnold and M. LaMarre assisted with the implementation. B.P. Bell conceived of the study and supervised all aspects of its implementation. All of the authors helped to conceptualize ideas, interpret findings, and review drafts of the article.
References
- 1.Barry MA, Gleavey D, Herd K, Schwingl PJ, Werner BG. Prevalence of markers for hepatitis B and hepatitis D in a municipal house of correction. Am J Public Health. 1990;80:471–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canada Dept of Health and Welfare. Seroepidemiologic study of hepatitis B and C viruses in federal correctional institutions in British Columbia. Can Dis Wkly Rep. 1990;52:265–266. [PubMed] [Google Scholar]
- 3.Weild AR, Gill ON, Bennett D, Livingstone SJM, Parry JV, Curry L. Prevalence of HIV, hepatitis B, and hepatitis C antibodies in prisoners in England and Wales: a national survey. Commun Dis Public Health. 2000;3:121–126. [PubMed] [Google Scholar]
- 4.Crofts N, Stewart T, Hearne P, Ping XY, Breschkin AM, Locarnini SA. Spread of bloodborne viruses among Australian prison entrants. BMJ. 1995;310: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz JD, Molitor F, Sun RK, et al. Prevalence and correlates of hepatitis C virus infection among inmates entering the California correctional system. West J Med. 1999;170:156–160. [PMC free article] [PubMed] [Google Scholar]
- 6.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89: 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. MMWR Morb Mortal Wkly Rep. 1991;40(RR-13):1–25. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Morb Mortal Wkly Rep. 2003;52(RR-1):1–44. [Google Scholar]
- 9.Taylor A, Goldberg D, Frischer M, Emslie J, Green S, McKeganey N. Transmission of HIV in prison: evidence of risk. BMJ. 1993;307:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbull P, Stimson GV. Drug use in prison. BMJ. 1994;308:1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird SM. Prevalence of drug injecting among prison inmates. Commun Dis Public Health. 2000;3: 308–309. [PubMed] [Google Scholar]
- 12.Rotily M, Delorme C, Obadia Y, Escaffre N, Galinier-Pujol A. Survey of French prison found that injecting drug use and tattooing occurred. BMJ. 1998; 316:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hull HF, Lyons LH, Mann JM. Incidence of hepatitis B in the penitentiary of New Mexico. Am J Public Health. 1985;75:1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decker MD, Vaughn WK, Brodie JS, Hutcheson RH, Schaffner W. The incidence of hepatitis B in Tennessee prisoners. J Infect Dis. 1985;152:214–217. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Hepatitis B outbreak in a state correctional facility, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:529–532. [PubMed] [Google Scholar]
- 16.Nainan OV, Cromeans TL, Margolis HS. Sequence-specific, single-primer amplification and detection of PCR products for identification of hepatitis viruses. J Virol Methods. 1996;61:127–134. [DOI] [PubMed] [Google Scholar]
- 17.Nainan OV, Stevens CD, Taylor PE, Margolis HS. Hepatitis B virus (HBV) antibody resistant mutants among mothers and infants with chronic HBV infection. In: Rizzetto M, Purcell RH, Gerin JL, Verme G, eds. Viral Hepatitis and Liver Disease. Turin, Italy: Edizioni Minerva Medica; 1997:132–134.
- 18.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1983;12:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center of Infectious Diseases. Disease burden from hepatitis A, B, and C in the United States, September 2002. Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/resource/dz_burden02.htm. Accessed July 15, 2005.
- 20.US Bureau of the Census. Census 2000 profile. Available at: http://www.census.gov/prod/2002pubs/c2kprof00-us.pdf. Accessed July 15, 2005.
- 21.US Dept of Justice. Prisoners in 2001. Available at: http://www.ojp.usdoj.gov/bjs. Accessed July 15, 2005.
- 22.Macalino GE, Vlahov D, Sanford-Colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Transmission of hepatitis B virus in correctional facilities—Georgia, January 1999–June 2002. MMWR Morb Mortal Wkly Rep. 2004;53:678–681. [PubMed] [Google Scholar]
- 24.Peters CJ, Purcel RH, Lander JJ, Johnson KM. Radioimmunoassay for antibody to hepatitis B surface antigen shows transmission of hepatitis B virus among household contacts. J Infect Dis. 1976;134:218–223. [DOI] [PubMed] [Google Scholar]
- 25.Bernier RH, Sampliner R, Gerety R, Tabor E, Hamilton F, Nathanson N. Hepatitis B infection in households of chronic carriers of hepatitis B surface antigen: factors associated with prevalence of infection. Am J Epidemiol. 1982;116:199–211. [DOI] [PubMed] [Google Scholar]
- 26.Management of hepatitis B virus in correctional facilities. Available at: http://www.ncchc.org/resources/statements/hepb.html. Accessed July 15, 2005.
- 27.Wolfe MI, Xu F, Patel P, et al. An outbreak of syphilis in Alabama prisons: correctional health policy and communicable disease control. Am J Public Health. 2001;91:1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canadian HIV/AIDS Legal Network. HIV/AIDS in prisons 2001/2002: prevention: condoms. Available at: http://www.aidslaw.ca/Maincontent/issues/prisons/e-info-pa4.htm. Accessed July 15, 2005.
- 29.Khan A, Goldstein S, Williams I, Bell B, Mast E. Opportunities for hepatitis B prevention in correctional facilities and sexually transmitted disease treatment settings. Antiviral Ther. 2000;5(suppl 1):22. [Google Scholar]
- 30.Goldstein ST, Alter MJ, Williams IT, et al. Incidence and risk factors for acute hepatitis B in the United States: 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–719. [DOI] [PubMed] [Google Scholar]
- 31.Pisu M, Meltzer MI, Lyerla R. Cost-effectiveness of hepatitis B vaccination of prison inmates. Vaccine. 2002;21:312–321. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Hepatitis B vaccination among high-risk adolescents and adults—San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51:618–621. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Hepatitis B vaccination of inmates in correctional facilities—Texas, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53:681–683. [PubMed] [Google Scholar]


