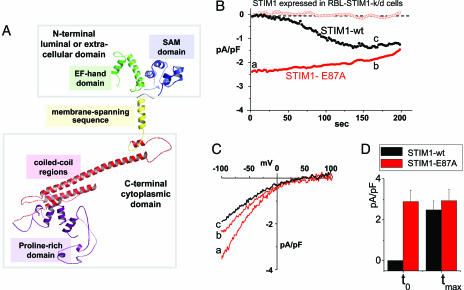
Fig. 2.
Expression of the E87A STIM1 mutant with decreased EF-hand Ca2+-binding affinity leads to constitutively active ICRAC in RBL cells. (A) Predicted 3D structure of STIM1 (57–591) generated by using phyre software (www.sbg.bio.ic.ac.uk/∼phyre). The domain structures shown, EF-hand, sterile-α motif (SAM), transmembrane-spanning region, coiled-coil regions, and proline-rich N terminus, were determined by similarity to proteins with known structure. (B) Either WT hSTIM1 or the E87A mutant with predicted lowered EF-hand Ca2+ affinity were reexpressed in rSTIM1 knockdown RBL cells. In contrast to the slow-developing store depletion-dependent current observed with STIM1 WT (black circles), ICRAC was constitutively active at the time of break-in (to) with STIM1 E87A-reexpressing cells (−80 mV, red closed circles; +80 mV, red open circles). (C) I–V profiles of STIM1 WT or STIM1 E87A measured at the time points indicated by a, b, and c in B are similar to endogenous ICRAC, with high positive reversal potential in 10 mM extracellular Ca2+, reflecting Ca2+ selectivity. (D) Comparisons of average current observed at to reveal significant differences between STIM1 WT(n = 3) and STIM1 E87A (n = 3), but not in maximal activation (tmax).