Abstract
An extending axon growth cone is subjected to attractant and repellent cues. It is not clear how these growth cones discriminate the two opposing forces and select their projection paths. Here, we report that in the Drosophila nerve cord the growth cones of longitudinal tracts are subjected to attraction by the Netrin-Frazzled pathway. However, the midline Slit neutralizes this pathway in a Robo-dependent manner and prevents Netrin-Frazzled-mediated attraction of longitudinal tracts. Our results suggest that the loss of a neutralizing effect on the Netrin-mediated attraction is responsible for the longitudinal tracts entering the midline in slit mutants as opposed to a loss of repulsion as is currently believed. This effect is not via a direct inhibition of Frazzled by Robo; instead, it is at a level downstream of Frazzled. Thus, the growth cones of longitudinal tracts subjected to two opposing forces are able to block one with the other and specify their correct lateral positioning along the midline.
IN both insects and vertebrates, chemorepellents and chemoattractants provide guidance cues to axons and regulate their pathfinding along stereotypical pathways toward their synaptic targets (reviewed in Tessier-Lavigne 1994; Goodman 1996; Van Vactor and Flanagan 1999; Harris and Holt 1999; Seeger and Beattie 1999). It is currently thought that membrane-bound receptors and secreted molecules function as either attractants or repellents mediating short-range and/or long-range signaling during this process. Thus, activities of axon repellents and axon attractants regulate the formation of commissures, the axon tracts that cross the midline and connect the two sides of the CNS, and longitudinal connectives, the axon tracts that travel along the nerve cord and connect different segments along the anterior-posterior axis.
Recently, much effort has been directed toward elucidating axon repulsion mediated by the Slit (Sli)-Roundabout (Robo) signaling (Ba-Charvet et al. 1999; Brose et al. 1999; Kidd et al. 1999; Li et al. 1999; Wang et al. 1999; Bashaw et al. 2000; Rajagopalan et al. 2000; Simpson et al. 2000) and axon attraction mediated by the Netrin (Net)-Frazzled (Fra) signaling (Harris et al. 1996; Kolodziej et al. 1996; Mitchell et al. 1996). For instance, it has been shown that growth cones that express Robo, Robo2, and Robo3, the receptors for Sli, will not enter and cross the midline where sli is expressed at high levels. Thus, in robo mutants axons freely cross and recross the midline whereas in sli mutants, axons that normally do not enter the midline freely do so but do not leave the midline after entering (Kidd et al. 1999; Bashaw et al. 2000; Rajagopalan et al. 2000; Simpson et al. 2000). The major conclusion from these studies is that a repellent interaction between Sli and the three different Robo proteins prevents the longitudinal pathways in the ventral nerve cord from projecting toward the midline (Kidd et al. 1999; Bashaw et al. 2000; Rajagopalan et al. 2000; Simpson et al. 2000). According to this conclusion, a gradient of Sli emanating from the midline interacts with these three different Robo receptors in a combinatorial manner to specify lateral positions of axon tracts in the longitudinal pathways (Rajagopalan et al. 2000; Simpson et al. 2000). High levels of Sli interact with Robo to specify the medial tract; intermediate levels of Sli interact with Robo, Robo 3, and low levels of Robo2 to specify the intermediate tract; and the lowest levels of Sli interact with Robo, Robo3, and high levels of Robo2 to specify the lateral tracts.
Several findings argue against the above model. For instance, a gradient of Sli extending from the midline has never been detected. More importantly, overexpression of sli at the midline does not alter the lateral positioning of longitudinal tracts (Kidd et al. 1999; our unpublished data), as one would expect for a repellent signal that emanates from the midline. Furthermore, robo2 mutants also exhibit a weak midline crossing of medial tracts phenotype (Rajagopalan et al. 2000), which is inconsistent with the proposed model since Robo2 is expressed only in the lateral tracts (Rajagopalan et al. 2000; Simpson et al. 2000). These results raise the possibility that the lateral positioning of longitudinal tracts by Sli-Robo signaling is much more complex than these studies suggest and involves additional pathways or mechanisms.
In Drosophila, Netrins (Net A and B) and their receptor, Fra (a member of the Deleted in Colorectal Cancer/UNC-40 family), first discovered and cloned and studied in Caenorhabditis elegans (Hedgecock et al. 1990; Ishii et al. 1992; Chan et al. 1996), mediate attraction of commissural growth cones toward the midline (Harris et al. 1996; Kolodziej et al. 1996; Mitchell et al. 1996; Stein and Tessier-Lavigne 2001). In net or fra mutant embryos the commissural tracts fail to cross the midline in a significant number of segments (Harris et al. 1996; Kolodziej et al. 1996; Mitchell et al. 1996). However, it is not known whether the Netrin-Fra-mediated attractant signaling has any role in the positioning of longitudinal tracts. Indeed, if these tracts are subjected to Net-mediated attraction, it is not clear how the longitudinal tract growth cones discriminate the two opposing forces (the Sli-Robo being the repellent one) and select their projection paths.
A previous study has shown that in stage 22 Xenopus spinal neurons binding of Sli to Robo leads to an interaction between the cytoplasmic domains of Robo and Fra, which in turn silences the Fra-Net-mediated attraction (Stein and Tessier-Lavigne 2001). We sought to determine if a similar interaction exists between Sli signaling and Net signaling, which then allows longitudinal tract growth cones to position along the nerve cord. In this article, we show that the growth cones of longitudinal tracts are subjected to attraction by the Net-Fra pathway. However, the midline Sli blocks this pathway in a Robo-dependent manner and prevents Net-Fra-mediated attraction. Thus, it appears that the loss of a neutralizing effect on Net-Fra-mediated attraction is responsible for the longitudinal tracts entering the midline in sli mutants as opposed to a loss of repulsion. Moreover, this effect is not via a direct inhibition of Fra by Robo as was shown in Xenopus using in vitro studies (Stein and Tessier-Lavigne 2001). Instead, it is at a level downstream of Fra. These results provide a novel insight into how the lateral tracts are positioned along the midline by two opposing cues.
MATERIALS AND METHODS
Stocks and genetics:
To analyze sli function, sli2, sliGA20 (null point mutations), sliE-158 (a hypomorphic P-element-insertion allele of sli; P-element insertion in the promoter region, see Rothberg et al. 1990), and a sli deficiency were used. For robo, robo4 (null allele) and a robo deficiency were used. For net A and net B double mutant, we used a small deficiency that eliminates the two genes and is well characterized for net phenotypes in previous studies (see Harris et al. 1996). For fra, the point mutants fra3 and fraGA957 and a deficiency for fra were used. Double mutants between net and sli or robo genes were constructed by standard genetics. The other stocks used were as follows: UAS-netA, UAS-netB, UAS-fra, UAS-sli, UAS-robo, elav-GAL4 (a pan-neural driver), sim-GAL4 (a midline driver), ptc-GAL4, arm-GAL4, UAS-GAL4, and UAS-Roboextra-Fraintra. Combinations between various UAS transgenes, GAL4 drivers, and mutants were constructed by standard genetics.
Ectopic expression of Sli:
To express sli in RP2, in rows of cells right above the RP2 cell body, in rows of cells immediately below aCC, and in rows of cells above pCC, we used the UAS-sli/UAS-GAL4 strategy with the ptc-GAL4 and arm-GAL4 as drivers (see supplementary Figure 1 at http://www.genetics.org/supplemental/ for the ptc and arm expression domains). UAS-sli was first introduced into UAS-GAL4 background. These flies were then crossed to either ptc-GAL4 or arm-GAL4. To have a continued clonal expression of Gal4, UAS-GAL4 was also introduced into these backgrounds (UAS-sli; UAS-GAL4; ptc- or arm-GAL4). UAS-GAL4 is activated initially by ptc- or arm-GAL4 and then clonally maintained by the UAS-GAL4 autoregulatory loop. For the pan-neural ectopic expression of sli, UAS-sli; UAS-GAL4 flies were crossed to elav-GAL4.
Antibodies and immunostaining:
Embryos were fixed and stained with the following antibodies: Fas II (1:5), BP102 (1:15), Sli (1:50), Eve (1:2000), Mab 22C10 (1:10), LacZ (1:3000), and Robo (1:50). For confocal microscopy, cy5 and FITC-conjugated secondary antibodies were used. For light microscopy, alkaline phosphatase or 3,3′-diamino-benzidine conjugated secondary antibodies were used. Mutant embryos were identified using blue balancers, marker phenotypes, or immunostaining (lack of positive staining). Please note that since a given stage can be as long as several hours or as short as 10 min, I have represented the age of the embryo in hours rather than stating the stage.
RESULTS
Netrin-Frazzled attractant signaling specifies the positioning of longitudinal tracts along the midline:
To determine if the Net-Fra pathway plays any role in the positioning of longitudinal tracts, we examined embryos mutant for these genes. Previous results have shown that in embryos mutant for fra the guidance of commissural axons is severely affected (Kolodziej et al. 1996). As shown in Figure 1C, these tracts are stalled and the nerve cord exhibits reduced or missing commissural tracts. Similar commissural defects were also observed in embryos mutant for netrins (Figure 1B). Moreover, the tracts are frequently projecting toward the periphery (Figure 1B, black arrowhead). In both net and fra mutants, commissural defects were observed in every segment of a mutant embryo (n = 30 embryos). In addition to the commissural defects, the longitudinal connectives were also affected in net or fra mutants. These tracts were positioned farther away from the midline in these mutants (n = 12 embryos) compared to wild type (see below). That this is caused by a reduction in the commissural tracts thereby compromising the cohesiveness or tension between tracts is unlikely since we observed several segments that lacked entire commissural tracts but were not any different from the regions where there was only a reduction in the commissural tracts (cf. Figure 3F). Finally, since the commissural tracts are not completely eliminated in net or fra mutants (Figure 1, B and C), there must be additional attractant cues from the midline, in addition to Netrins, that the commissural tracts respond to in order to project toward the midline.
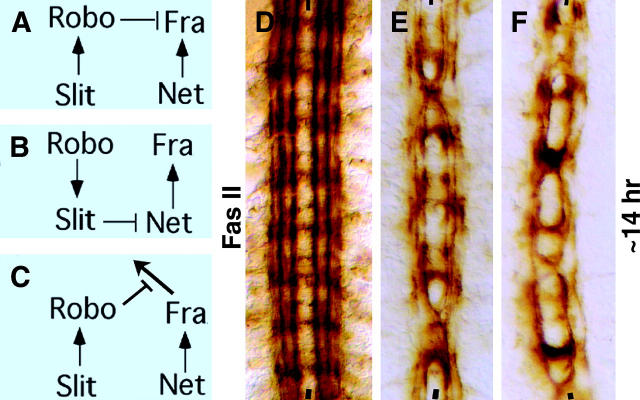
Figure 1.—
Netrin-Frazzled signaling attracts longitudinal axon tracts toward the midline. Anterior side is up; midline is marked by vertical lines. Embryos are ∼14 hr old. WT, wild type. Embryos in A–C are stained with BP102; scale bar for A–C is given in A. (A) Wild type. BP102 stains the anterior commissure (AC), the posterior commissure (PC), and the longitudinal connectives (LC). (B and C) In net and fra mutants, the connectives are farther apart and the commissures are often missing, very thin (white arrowhead), or project outward (black arrowhead). Embryos in D–I are stained for Fas II; scale bar for D–F is given in D. (D) Wild-type embryo. Fas II stains pCC (arrow) and its projection (long arrow), which pioneers the medial tract. (E and F) net and fra mutant embryos. The projections from pCC are farther apart than in wild type. (G) Wild-type embryo. Fas II stains three major longitudinal tracts, medial (M), intermediate (I), and lateral (L). (H and I) net and fra mutants. These Fas II-positive pathways are farther away from the midline and are often disorganized or missing in regions along the tract (see also Table 1).
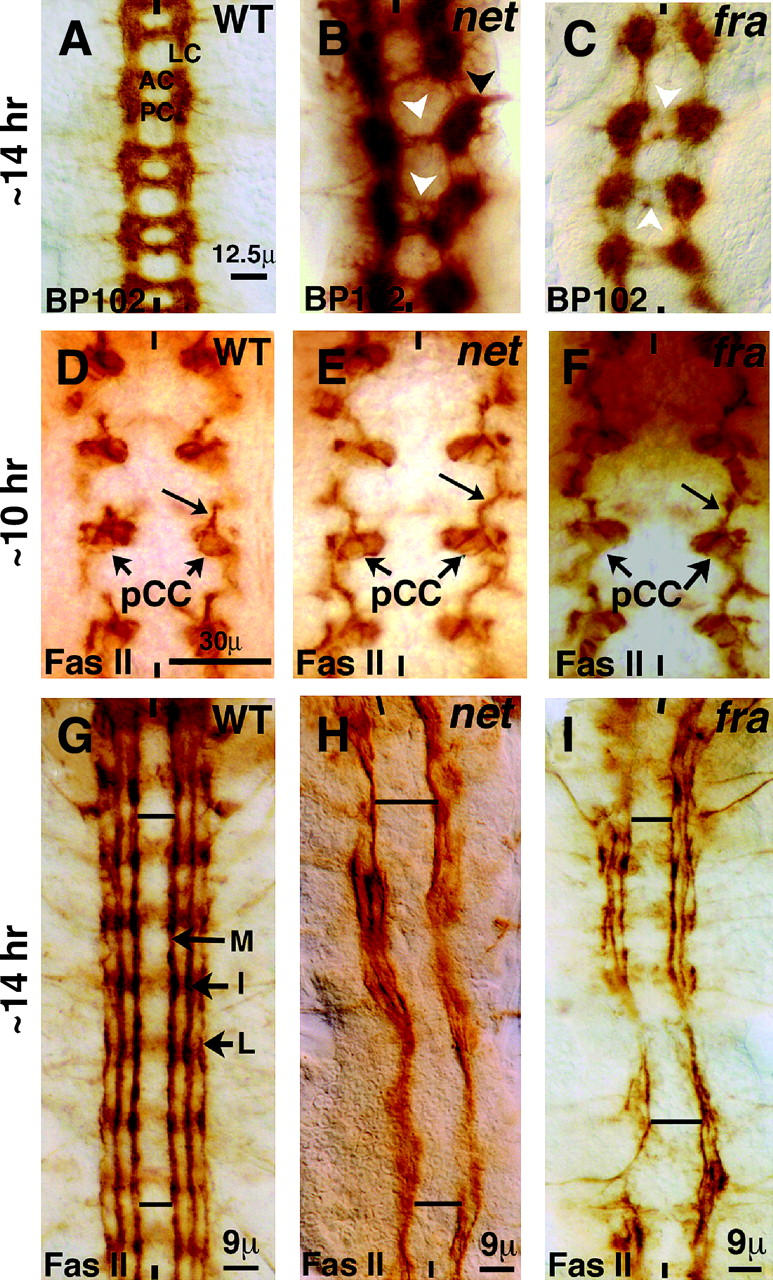
Figure 3.—
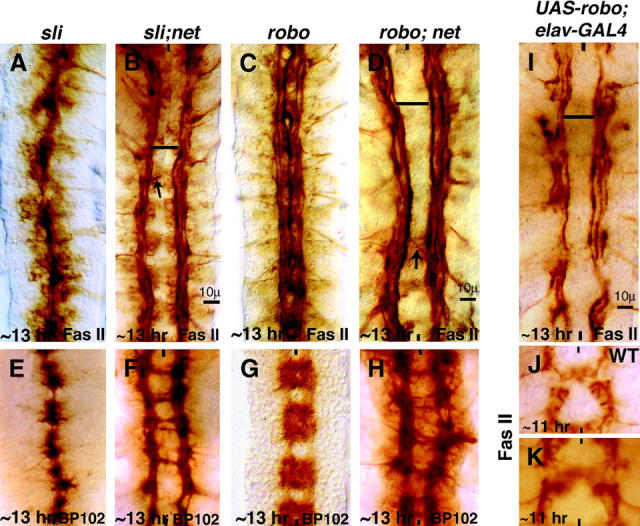
netrin phenotype is epistatic to the sli and robo phenotypes. Anterior side is up; midline is marked by vertical lines. Embryos are ∼14 hr old. Embryos in A–D are stained with Fas II. While in sli the longitudinal tracts are collapsed at the midline (A), in the sli; net double mutant (B), they are farther away from the midline as in a net single mutant. Similarly, while in robo (C) the medial tracts abnormally enter the midline, in the robo; net double mutant (D), they no longer enter the midline and the phenotype is the same as net single mutant. The distance between two medial tracts across the midline in wild type is ∼10 μm and is indicated by a bar in B, D, and I. Arrows in B and D indicate midline crossing of longitudinal tracts. Embryos in E–H are stained with BP102. BP102 staining also shows that the net phenotype is epistatic to the sli or robo phenotypes. I–K are stained for Fas II.(I) An ∼13-hr-old UAS-robo/+; elav-GAL4/elav-GAL4 embryo. (J) An ∼11-hr-old wild-type embryo. (K) An ∼11-hr-old UAS-robo/+; elav-GAL4/elav-GAL4 embryo (see text for details).
Previous work in C. elegans showed that Net determines the placement of longitudinal axons with respect to the midline (Ren et al. 1999). However, it was not known if the Net-Fra signaling also exerts an attraction on the well-studied fasciclin II (Fas II)-positive longitudinal tracts in the Drosophila embryo. We sought to examine this by staining net and fra embryos with anti-Fas II (mAb1D4). As shown in Figure 1G, in wild type Fas II stains three major longitudinal pathways: the medial pathway (closest to the midline and formed first), the lateral pathway (farthest from the midline), and the intermediate pathway (between the medial and the lateral). One of the pioneering axons for the medial tract is from the interneuron pCC. Fas II stains both pCC and its axon projection at an earlier stage of development (Figure 1D). In wild type the distance between medial tracts is progressively reduced from ∼30 μm in 10-hr-old embryos to ∼9 μm in 14-hr-old embryos (Table 1). When we examined 10-hr-old net or fra mutant embryos, we found that, compared to those in wild type, the tracts from pCC neurons were projected farther away from the midline (Figure 1, E and F), positioned ∼40 μm and ∼37 μm, respectively (Table 1); however, the difference in the position of the pCC neuron in net and fra mutants and in wild type was minimal. When we examined ∼14-hr-old embryos, we found that in net and fra mutants the medial tracts were positioned ∼20 μm and ∼18 μm apart, respectively, while in wild type the medial tracts were ∼9 μm apart (Figure 1, H and I; Table 1). These results suggest that Net, which is expressed in the midline, and Fra, which is present in the growth cones, provide an attractant signaling cue for the lateral positioning of medial tracts on either side of the midline.
TABLE 1.
The distance between the two medial tracts across the midline at three different embryonic developmental points in wild-type and mutant backgrounds
| Age (hr) | Wild type (μm) | net (μm) | fra (μm) | net;sli (μm) | net; robo (μm) |
|---|---|---|---|---|---|
| 10 | 30 ± 1 | 40 ± 3 | 37 ± 3 | 41 ± 3 | 39 ± 3 |
| 11.5 | 19 ± 0.5 | 31 ± 3 | 28 ± 2 | 29 ± 3 | 29 ± 2 |
| 14 | 9 ± 0.5 | 20 ± 2 | 18 ± 1 | 21 ± 2 | 21 ± 3 |
The embryos were stained with Fas II antibody. The values for each genotype and each time point are averages taken from 12 different embryos and the distance was measured from each embryo in at least two different points along the nerve cord. Note that the distance between the two medial tracts across the midline is decreasing with embryonic development.
The intermediate and lateral tracts in net or fra mutants did not appear to be farther away from the midline than those in wild type. However, these tracts were broken and often collapsed on each other. Since Netrins also promote axon growth, this intermittent breaking of the two lateral tracts (but interestingly not the medial tract) might be due to an effect of loss of Net activity on axon growth. Moreover, the nerve cord in the mutants was much narrower compared to that in wild type. It may be that some neurons from the lateral neuroblasts are missing in net or fra mutants, resulting in a much narrower nerve cord. This can cause broken nerve tracts and might also correct the abnormal lateral positioning of the longitudinal tracts to some extent in these mutant embryos.
We further tested the possibility that the Net-Fra signaling system influences the lateral positioning of longitudinal tracts along the midline. If Net attracts the longitudinal tracts toward the midline, an overexpression of Net in the midline should attract longitudinal tracts toward the midline and either make these tracts collapse at the midline or, at the minimum, cause a shift in the lateral positioning of longitudinal tracts closer to the midline. Indeed, when net was overexpressed in the midline from UAS-net (net A or net B) with sim-GAL4 driver in wild-type background (each in single copy), it resulted in the collapse of the longitudinal tracts at the midline (Figure 2, C and D; two copies each causes a stronger collapsing of longitudinal tracts, data not shown). This phenotype was observed in ∼40% of the hemi-segments (n = 336 hemi-segments). Furthermore, the lateral positioning of the tracts in places where they have not collapsed at the midline is shifted more toward the midline (Figure 2D). Furthermore, we also overexpressed net in the midline in a sli hypomorphic mutation, sliE158. This is a P-element-insertion allele (Kidd et al. 1999) and the longitudinal tracts are not as severely affected (Figure 2E) as in sli null embryos (Figure 3A). Overexpression of net at the midline in this sli hypomorphic background (UAS-net and sim-GAL4, each in single copy) caused a significant enhancement of the phenotype, and the longitudinal tracts of these embryos appeared similar to strong loss-of-function sli mutant embryos (Figure 2F). In summary, these results argue that Net signaling plays an attractant role in the lateral positioning of longitudinal tracts, in particular the medial tract along the midline.
Figure 2.—
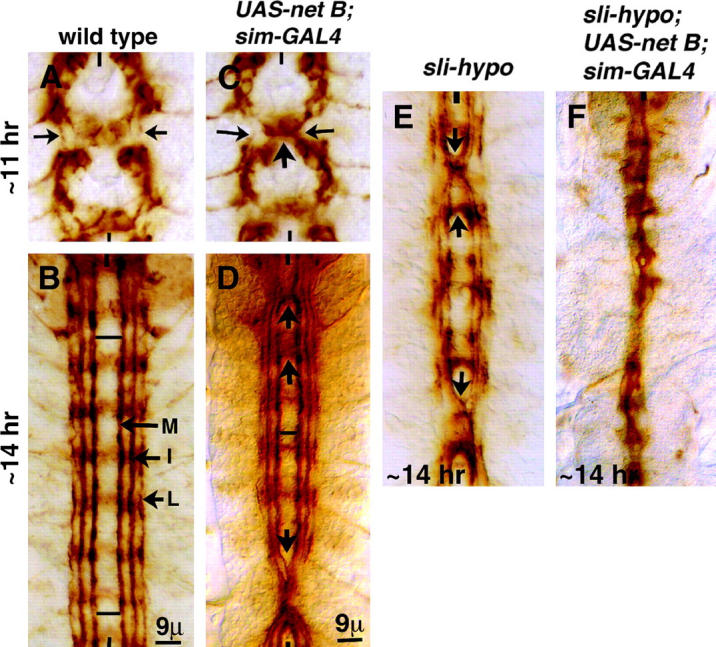
Overexpression of Net at the midline attracts longitudinal tracts toward the midline. Anterior end is up; midline is marked by vertical lines. Embryos are stained for Fas II. (A) Wild-type ∼11-hr-old embryo showing the projections from pCC neurons (arrows). (B) Wild-type ∼14-hr-old embryo showing the three major Fas II tracts, medial (M), intermediate (I), and lateral (L). (C) An ∼11-hr-old UAS-net B/+; sim-GAL4/+ embryo. The projections from two pCC neurons on either side of the midline are attracted toward the midline (arrows). (D) An ∼14-hr-old UAS-net B/+; sim-GAL4/+ embryo. Arrows indicate the midline crossing of longitudinal tracts or their collapsing at the midline. (E) In an ∼14-hr-old sli hypomorphic mutant embryo, mostly the medial tracts cross the midline (arrows). (F) An ∼14-hr-old UAS-net B/sim-GAL4; sli-hypo/sli-hypo embryo.
netrin/frazzled phenotype is epistatic to the sli/robo phenotype:
In sli null mutant embryos, the longitudinal tracts are collapsed at the midline in a fully penetrant manner (Kidd et al. 1999). This can be observed with Fas II and BP102 staining (Figure 3, A and E). When two mutants have opposing phenotypes, genetic epistasis studies have been most revealing in determining the hierarchy of gene activity and the relationship between the two genes in regulating a given process (Noordermeer et al. 1994; Siegfried et al. 1994; Bhat 1996; Bhat and Schedl 1997; Bhat et al. 2000). Since net and sli have opposing mutant phenotypes, we sought to determine the epistatic relationship between these two genes. We generated sli; net double mutants and examined the positioning of longitudinal tracts in these embryos. As shown in Figure 3, while in sli the tracts are collapsed at the midline (Figure 3, A and E), in double mutants the positioning of tracts was as in net single mutants (Figure 3, B and F; see Table 1). This phenotype was fully penetrant as in net single mutant embryos (n = −30 embryos). This result indicates that the net phenotype is epistatic to the sli phenotype. One plausible interpretation of this result is that Net functions downstream of Sli and not in parallel (see also below). That is, in sli mutants Net at the midline exerts a strong attraction on growth cones, causing the tracts to collapse at the midline, whereas in the double mutant, since there is no Net-mediated attraction, growth cones no longer are attracted to the midline even when there is no Sli. In molecular terms, our above results suggest that Sli neutralizes Net-mediated attraction of growth cones toward the midline.
If Sli functions downstream of Net, we would have expected to observe a sli mutant phenotype in the sli; net double mutants. The third possibility is that Sli and Net function in a parallel pathway. In a parallel pathway, Net will attract lateral tract growth cones whereas Sli will repel the same growth cones independently of each other. Here, we expect to observe either an intermediate or a wild-type (or close to wild-type) lateral positioning of the longitudinal tracts in the double mutant. However, as shown in Figure 3B, the lateral positioning of longitudinal tracts is the same in the double mutant and the net single mutant. Therefore, while it is still possible that the two pathways are independent of each other, we believe that the neutralization model described above is probably the likely scenario (see below).
We next asked if the interaction between Robo and Sli is necessary to antagonize Net-mediated growth cone attraction from the midline. In robo mutants, the medial tracts inappropriately collapse at the midline (Figure 3, C and G). We generated robo; net double mutants and examined which of the phenotypes is epistatic in these embryos. As shown in Figure 3, D and H, these embryos also exhibited a net phenotype (see also Table 1), indicating that Robo and Sli interaction is necessary for Sli to oppose Net-mediated attraction. We also point out that, while longitudinal axons cross the midline in net single mutants in <1% of the hemi-segments (n = 1100; data not shown), the frequency of longitudinal axons crossing the midline is slightly enhanced in both sli; net (3.5% of the hemi-segments, n = 840) and robo; net (3%, n = 840) double mutants (arrows in Figure 3, B and D). This suggests that the direct Sli-Robo-mediated repulsive signaling (Kidd et al. 1999) plays a contributory role in preventing longitudinal tracts from crossing the midline. We also examined robo2; net and robo3; net double-mutant embryos and in each of these cases, the net phenotype was epistatic (data not shown). This argues that both Robo2 and Robo3 also function via neutralizing the attraction by Net (see discussion).
Finally, since the neutralizing effect on Net-mediated attraction by Sli is Robo dependent, we reasoned that an excess of Robo (robo gain of function) in wild-type background should also lead to a loss of Net attraction. We expressed robo from a UAS-robo transgene in a pan-neural fashion using the elav-GAL4 driver and examined the positioning of longitudinal tracts in these embryos. As shown in Figure 3, I and K, in embryos overexpressing robo in a pan-neural fashion, the longitudinal tracts were positioned much more laterally and the phenotype resembled that of net mutant embryos (n = 12 embryos). This phenotype was the opposite of the one induced by the overexpression of net at the midline (see Figure 2, C and D).
Ectopic Slit fails to repel Robo-positive growth cones:
Given the above possibility, we sought to further examine the role of direct repulsive interaction between Sli and Robo within the nerve cord and to determine if Sli can repel growth cones of Robo-positive pCC (an interneuron) and two motoneurons, aCC and RP2, when expressed ectopically in their projection paths. Specifically, we sought to reorient the longitudinal projection of pCC and ipsilateral and postero-lateral projections of RP2 and aCC [loss of Sli or Robo activity causes pCC to project toward the midline in a fully penetrant manner (Kidd et al. 1999) whereas the abnormal projection of aCC and RP2 toward the midline is observed in ∼15% of the hemi-segments (see Wolf and Chiba 2000)] by ectopically expressing Sli using the UAS-GAL4 system in front of their projection paths. The drivers patched (ptc)-GAL4 (Figure 3B) and armadillo (arm)-GAL4 (Figure 4c) were used to ectopically express Sli from a UAS-sli transgene (see supplementary information at http://www.genetics.org/supplemental/ for the expression pattern of ptc and arm). We also introduced a UAS-GAL4 transgene to maintain sli expression at high levels continually prior to and during axonogenesis following the initial induction by an autoregulatory loop (Hassan et al. 2000). In these experiments, the growth cones of aCC and RP2 ignored the ectopic Sli stripe and projected normally (Figure 4, e, h, f, and i; b and c show levels of ectopic Sli at about the same time that RP2 begins to grow an axon; n = 336 hemi-segments). The levels of ectopic Sli in these stripes are as high as in the midline (Figure 4, b and c) (the ptc and arm drivers are active as early as ∼4 hr of development, see Bhat 1996; see supplementary results at http://www.genetics.org/supplemental/). Similarly, the growth cone of pCC also projected normally (Figure 4, k and j; n = 336 hemi-segments) as in wild type, contrary to the expectation that the ectopic Sli stripe would repel its growth cone and we would observe breaks in the medial tracts with anti-Fas II staining (cf. Schindelholz et al. 2001). These results indicate that Sli-Robo interaction either does not always lead to a repulsive output or requires additional inputs that are not available outside of the midline. On the other hand, these results also support the possibility that Sli-Robo interaction regulates positioning of axon tracts mostly by neutralizing Net-mediated attraction.
Figure 4.—
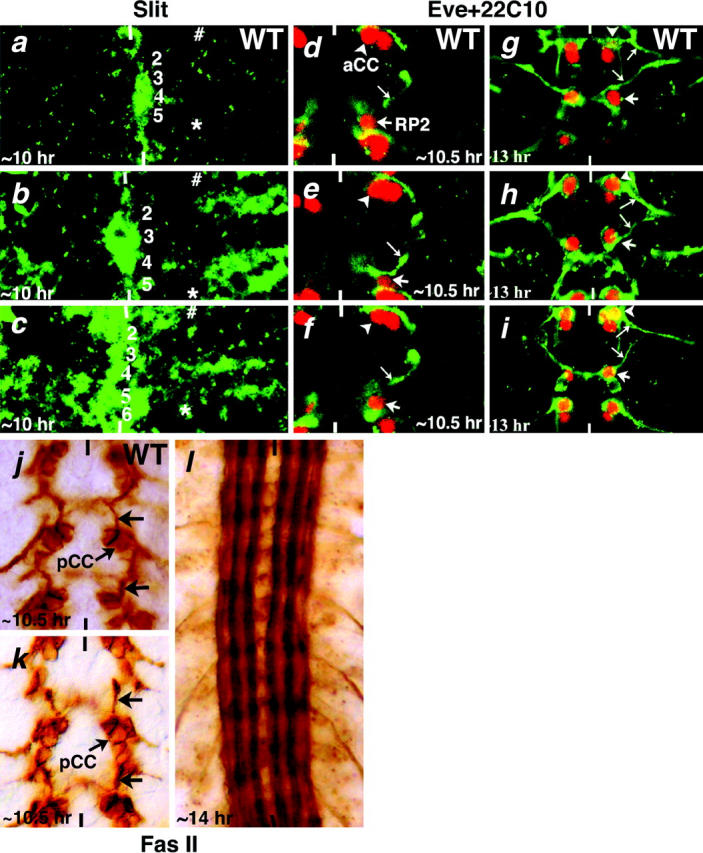
Striped ectopic expression of sli in front of the projection paths of RP2, aCC, and pCC does not alter their projection pattern. Embryos in a–c are stained for Sli. In d–i, embryos are double stained for Eve (red, nuclear) and 22C10 (green, membrane). Embryos in j–l are stained with anti-Fas II. Anterior end is up; midline is indicated by the vertical lines. Numbers along the midline in a–c indicate rows of neuroblasts. In a–c, the positions of aCC and RP2 neurons during the time of axonogenesis are marked by a number sign and an asterisk, respectively. Large arrows mark the RP2, small arrows indicate axon projection from RP2 and aCC, and arrowheads indicate aCC. (a) Wild-type embryo. Sli is only in the midline cells. (b) UAS-sli; UAS-GAL4; ptc-GAL4 embryo. Sli is also present in rows of cells above RP2 and above pCC. (c) UAS-sli; UAS-GAL4; arm-GAL4 embryo. Sli is present in rows above RP2 and below aCC. (d) Wild-type embryo. Projections from aCC and pCC neurons have not yet fasciculated with each other. (e and f) UAS-sli; UAS-GAL4; ptc-GAL4 and UAS-sli; UAS-GAL4; arm-GAL4 embryos. In e and f, the axon projections of RP2 and aCC are not yet fasciculated but they are similar to wild type and not repulsed by the ectopic Sli stripes. (g) Wild-type embryo. Both aCC and RP2 projections have fasciculated together. (h and i) UAS-sli; UAS-GAL4; ptc-GAL4 and UAS-sli; UAS-GAL4; arm-GAL4 embryos. Both aCC and RP2 have normal projection patterns and are not repulsed by the ectopic Sli stripes. Note that we could not simultaneously examine these embryos with Sli and 22C10 antibodies since both these primary antibodies are mouse antibodies. (j) An ∼10.5-hr-old wild-type embryo showing pCC and its projection. (k) An ∼10.5-hr-old UAS-sli; UAS-GAL4; ptc-GAL4 embryo. The projection from pCC is not affected. (l) An ∼14-hr-old UAS-sli; UAS-GAL4; ptc-GAL4 embryo. No breaks in the longitudinal tracts were observed.
It was possible that ectopic expression of sli in stripes causes a downregulation of Robo; thus, these growth cones cannot respond to Sli. However, expression of Robo was unaffected in these embryos (data not shown). Moreover, a robo-like phenotype was also not observed, as one would have expected if Robo were downregulated. Since these UAS-sli transgenes rescue sli mutant phenotype (data not shown; see Kidd et al. 1999), it is unlikely that ectopic Sli produced by these transgenes is nonfunctional.
The blocking of Net-mediated attraction of growth cones by Sli-Robo signaling occurs downstream of Frazzled:
The blocking of Net-mediated attraction of growth cones by Sli-Robo can occur in several ways. Previous results have shown that, in stage 22 Xenopus spinal neurons, binding of Sli to Robo leads to an interaction between the cytoplasmic domains of Robo and Fra, which in turn silences the Fra-Net-mediated attraction (Stein and Tessier-Lavigne 2001; see Figure 5A). If this scenario is correct, availability of an excess of Fra should overcome the inhibitory interaction between Robo and Fra and the neutralizing effect on Net-mediated attraction and therefore should lead to a collapsing of the longitudinal tracts at the midline. Therefore, initially we overexpressed fra in wild-type background in a pan-neural manner. This did not result in any axon guidance phenotype (Figure 5D; n = 30 embryos). Next, we overexpressed fra in the hypomorphic sliE-158 mutant background (in this allele a P-element is inserted in the 5′ region of the gene; thus, only the expression of the gene is affected but not the protein itself). This should lead to an enhancement of the collapsing of longitudinal axon tracts at the midline of sli hypomorphic mutants. However, no such enhancement was observed (Figure 5F; n = 30 embryos). Furthermore, increasing the copy numbers of fra using a duplication chromosome also had no effect (n = 30 embryos). These are negative results; however, since the fra transgene rescues the fra mutant phenotype when expressed using the elav-GAL4 driver (each in single copy; see also Keleman and Dickson 2001), this transgene does produce functional Fra protein and the negative results are likely to be meaningful.
Figure 5.—
Interaction between Robo, Sli, Net, and Fra during the positioning of longitudinal and commissural tracts. There are at least three alternative possibilities for the interaction of these proteins. In the first scenario (A), Robo prevents Fra-Net interaction in a Sli-dependent way. In the second (B), Sli prevents Net-Fra interaction in a Robo-dependent way. In the third (C), Robo neutralizes the Fra-mediated attractant signaling at the downstream level. These possibilities are examined in D and F (see text for details). Embryos in D–F are stained for Fas II. Anterior end is up; midline is marked by vertical lines. (D) An ∼14-hr-old UAS-fra; elav-GAL4 embryo. Pan-neural expression of Fra has no effect. (E) An ∼14-hr-old sli hypomorphic embryo. (F) An ∼14-hr-old sli-hypo/sli-hypo; UAS-fra/elav-GAL4 embryo. There was no worsening of the phenotype in sli hypomorphic mutant CNS with pan-neural overexpression of Fra.
A previous study found that Sli binds to Net in vitro (Brose et al. 1999). It is therefore possible that binding of Robo to Sli leads to a physical interaction between Sli and Net and silencing of Net-Fra interaction (Figure 5B). However, this possibility, in vivo, seems unlikely for two reasons. First, overexpression of sli at the midline fails to cause any phenotype (see Kidd et al. 1999; our unpublished results). In the above scenario, it is expected that too much of Sli would neutralize the attraction and the tracts will be positioned farther away from the midline. Second, the pan-neural overexpression of fra in wild-type and sli hypomorphic mutant backgrounds did not result in the attraction of axon tracts toward the midline (Figure 5, D and F; n = 12 embryos). Under the above scenario, there is more of Net available in the sli mutant and, thus, overexpression of fra should have caused an enhancement of the midline collapsing defect in sli mutant embryos. These results, therefore, argue that Robo signaling interferes indirectly with the Net-Fra signaling. That is, an interaction between Sli with Robo leads to an interference of Net-Fra-mediated attraction downstream of Fra (Figure 5C). Therefore, overexpression of either Fra or Sli need not affect the positioning of axon tracts.
The effect of Roboextra-Fraintra chimeric protein on longitudinal tracts is dependent on the presence of Net activity:
In a previous reciprocal domain-swapping experiment, Bashaw and Goodman (1999) found that expression of a chimeric protein between the extracellular domain of Fra and the intracellular domain of Robo (Fraextra-Robointra) caused a repulsion of commissural tracts. On the other hand, expression of a chimeric protein between the extracellular domain of Robo and the intracellular domain of Fra (Roboextra-Fraintra) caused midline crossing of longitudinal tracts and recrossing of commissural tracts. We sought to determine if the midline crossing of longitudinal tracts in Roboextra-Fraintra embryos is dependent on the presence of an intact Net activity. In the direct Sli and Robo repulsive signaling scenario, the clear prediction is that the sli-like phenotype of Roboextra-Fraintra would be epistatic to the net phenotype. In our model, we would expect the net phenotype to be epistatic. We generated embryos of the genotype UAS-Roboextra-Fraintra/elav-GAL4; UAS-Roboextra-Fraintra/+; net/Y and examined their longitudinal axon guidance phenotype by anti-Fas II staining. These embryos had the net phenotype (Figure 6B; n = 12 embryos) indicating that the net phenotype is epistatic to the Roboextra-Fraintra phenotype (Figure 6A).
Figure 6.—
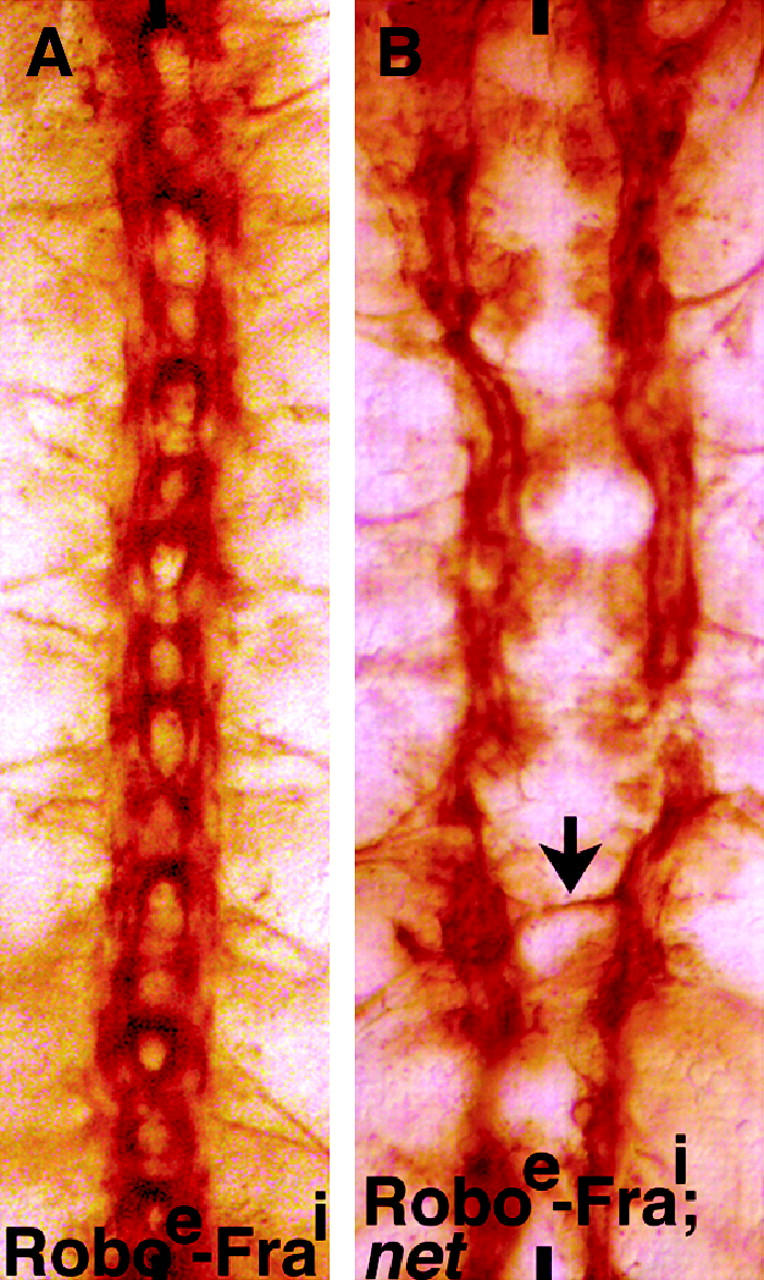
Growth cone attraction mediated by the chimeric protein between Robo and Fra is net dependent. Embryos are stained with anti-Fas II. Anterior end is up; midline is marked by vertical lines. (A) UAS-Roboextra-Fraintra/elav-GAL4; UAS-Roboextra-Fraintra/+ embryo. (B) UAS-Roboextra-Fraintra/elav-GAL4; UAS-Roboextra-Fraintra/+; net/Y embryo. See also discussion, Figure 7, and its legend for more details.
DISCUSSION
Sli-Robo signaling neutralizes attraction of growth cones of the longitudinal tracts by Net-Fra and specifies their lateral position:
The precise lateral positioning of the longitudinal axon tracts along the VNC in the Drosophila embryo is a very important paradigm to study how growth cones navigate in a diverse environment and find their synaptic targets. Several previous studies have concluded that a direct combinatorial repulsive interaction between Sli secreted from the midline glial cells and the three different Robo proteins on growth cones specifies the lateral positioning of longitudinal tracts along the midline (Bashaw and Goodman 1999; Kidd et al. 1999; Rajagopalan et al. 2000; Simpson et al. 2000). However, the results described in this article bring out another possibility that the Sli-Robo signaling neutralizes the attractant cue mediated by the Net-Fra signaling to specify the lateral positioning of longitudinal pathways (Figure 7A). Thus, the inappropriate entry of longitudinal growth cones and their collapsing at the midline in sli mutants could be due to the loss of silencing of attraction by Net-Fra signaling. Since Robo is downregulated in commissural tracts (Tear et al. 1996) and therefore there is no silencing of the Net-Fra attractant cue, Net-Fra signaling is able to mediate attraction of commissural axons toward the midline (Figure 7A).
Figure 7.—
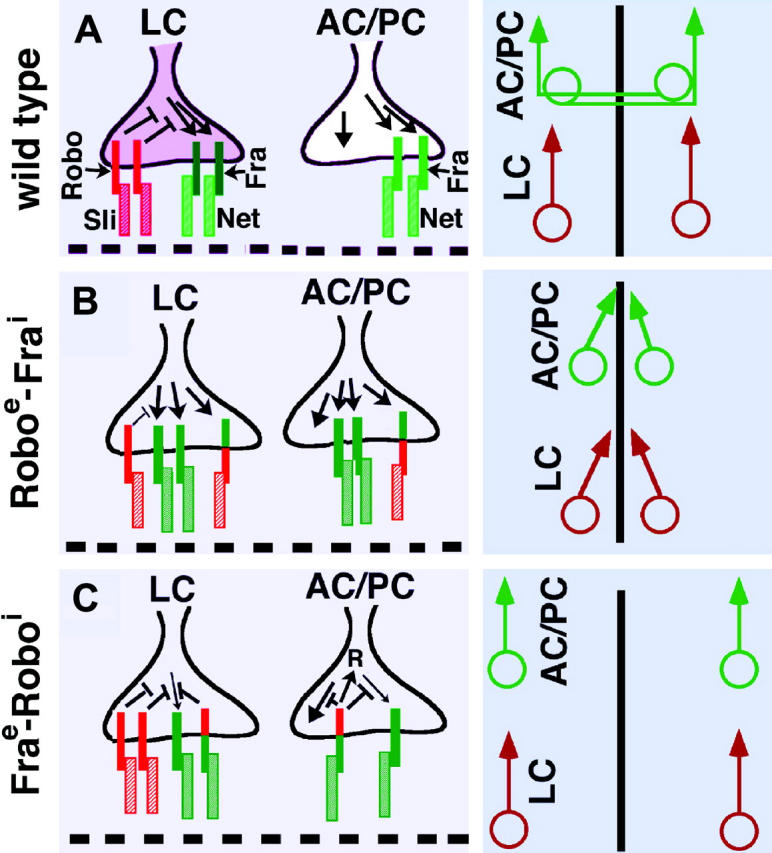
Interaction between Sli-Robo and net-Fra signaling pathways. (A–C) Schematics of growth cone of longitudinal connective (LC), anterior commissure (AC), and posterior commissure (PC) along with localization of Robo (solid red bar), Sli (hatched red bar), Net (light green bar), and Fra (dark green bar). Midline is marked by a dashed line. (A) In LC, Robo-Sli interaction blocks Net-Fra pathway downstream of Fra. In AC and PC where growth cones project toward the midline, the growth cones lack Robo; thus, Net-Fra pathway is able to mediate attraction. Additional attractant cues must also exist since net or fra mutant embryos do not display a complete commissureless phenotype. (B) In LC, with the pan-neural overexpression of Roboextra-Fraintra, there is a reduction in the neutralizing effect of Net-Fra attraction by Sli-Robo due to the dominant negative effect of the chimeric protein and an increase in the attraction mediated by the Fraintra causing a sli-like phenotype. In AC and PC, there is an enhancement of the attraction due to the chimeric Robo-Fra signaling. (C) In LC, with the pan-neural overexpression of Fraextra-Robointra, the Net-Fra-mediated attraction is negatively affected in two ways: a dominant negative effect and an increase in the neutralizing effect of the attraction. This results in the axon tracts positioned farther away. In AC/PC, not only does the ectopic expression of the chimeric protein neutralize the Net-Fra attraction, but also the Robointra neutralizes the additional attractant cues since the commissureless phenotype is very strong. The commissureless phenotype may also be due to a significant contribution from a Sli-Robo-mediated direct repulsive signaling (see text).
What is the evidence to support the above model? First, loss of function for net or fra causes a more lateral shift in the position of the longitudinal tract (Figure 1), indicating that Net-Fra exerts an attractant force on these tracts. Second, overexpression of net at the midline causes an inappropriate projection of longitudinal growth cones toward the midline and the collapsing of the lateral tracts at the midline (a similar overexpression of sli at the midline has no consequence, see also Kidd et al. 1999). Moreover, such an expression of net in a sli hypomorphic mutant background enhances the midline collapsing of lateral tracts (Figure 2). These indicate that enhanced levels of Net can overcome silencing by Sli-Robo signaling. Third, the net phenotype was epistatic to the sli phenotype and the longitudinal tracts were more laterally placed as in net mutant embryos in net; sli or net; robo or fra; sli and fra; robo double-mutant combinations (Figure 3). When there is no attraction mediated by Net-Fra signaling, loss of sli or robo does not cause longitudinal growth cones to inappropriately enter the midline. Therefore, Sli-Robo signaling is necessary to prevent these tracts from entering the midline only when there is attraction by Net-Fra. These epistatic results also suggest that this is not a parallel pathway, but it is a linear pathway where Net is downstream of Sli. Fourth, ectopic expression of Sli in front of the growth cones of Robo-expressing neurons fails to prevent these growth cones from projecting normally toward their targets (Figure 4). For example, pCC, which sends out one of the pioneering axons for the medial longitudinal tract, normally extends its growth cone despite having ectopic Sli in front of it. Similarly, projections from an RP2 or aCC (motoneurons) also fail to respond to ectopic Sli. All three neurons express Robo and are affected in sli mutants. Moreover, the transgene used in these ectopic expression experiments can rescue the loss-of-function sli mutant phenotype (Kidd et al. 1999). Therefore, the inability of these growth cones to respond to ectopic Sli argues against a direct repulsive scenario and supports the model where Sli-Robo signaling specifies the lateral position of longitudinal tracts by silencing Net-Fra-mediated attraction.
Finally, expression of a chimeric protein between the extracellular domain of Robo and the intracellular domain of Fra (Roboextra-Fraintra) causes midline crossing of longitudinal tracts (Bashaw and Goodman 1999). If the direct repulsion scenario is correct, this chimeric protein should convert the direct repulsion into a direct attraction (as was proposed, see Bashaw and Goodman 1999). Therefore the Roboextra-Fraintra phenotype should be epistatic to the net phenotype. But expression of this chimeric protein in a net mutant background suppressed the inappropriate midline crossing of longitudinal tracts and these embryos had the net phenotype (Figure 6).
How can we explain the midline crossing of longitudinal tracts and recrossing of commissural tracts observed with the expression of a Roboextra-Fraintra? In the longitudinal connectives (LCs), the chimeric protein contributes to the attraction mediated by endogenous Net-Fra signaling and, as a result, the silencing effect of endogenous Sli-Robo signaling is overcome (Figure 7B). In the absence of endogenous Net activity in Roboextra-Fraintra; net embryos, the attraction mediated by the Roboextra-Fraintra chimeric protein is neutralized by the endogenous Sli-Robo signaling. Furthermore, the two chimeric proteins have strong dominant negative effects (Bashaw and Goodman 1999). Thus, while it has been claimed previously that this chimeric protein actively attracts axons to the midline using the attractive activity of the Fra intracellular domain, it is also possible that the effect of the fusion protein reflects titration of Slit by the Robo extracellular domain, without the Fra moiety doing anything active at all. If that is the case, the fusion protein is acting as a dominant negative and behaving as a sli hypomorphic mutation and the epistatic result between net and this chimeric line does not add any new information.
On the other hand, Bashaw and Goodman (1999) argue that the dominant negative effects of the chimera are minimal. If this is true, at the minimum, the dominant negative effect will contribute to the inappropriate midline crossing phenotype observed in Roboextra-Fraintra embryos. This possibility is further supported by the finding that overexpression of Fra does not lead to any guidance phenotypes. Furthermore, the same dominant negative effect will also reduce the ability of endogenous Sli signaling to neutralize the attraction mediated by the Roboextra-Fraintra protein in Roboextra-Fraintra; net embryos. (The above sli-like phenotype in Roboextra-Fraintra embryos further argues against a direct Sli-Net binding scenario since such a binding would create a loss of attraction and thus a net or fra-like phenotype.) In anterior commissure (AC) and posterior commissure (PC) where growth cones project toward the midline (Figure 7B), the growth cones lack Robo; thus, the Net-Fra pathway is able to mediate attraction. Additional attractant cues must also exist since net or fra mutant embryos do not display a complete commissureless phenotype.
Expression of a chimeric protein between the extracellular domain of Fra and the intracellular domain of Robo (Fraextra-Robointra) causes a more lateral positioning of longitudinal tracts (Figure 7C; see Bashaw and Goodman 1999). This is expected since the chimeric protein in this case will contribute to a more efficient silencing of the Net-Fra attraction (Figure 7C). Again, since this chimeric protein also has strong dominant negative effect (Bashaw and Goodman 1999), the attraction by the endogenous Net-Fra signaling is likely to be weaker in embryos expressing pan-neural Fraextra-Robointra compared to wild type. In the longitudinal tracts (LC), this reduced attraction is compounded by a silencing of the attraction mediated by the intracellular domain of Robo, resulting in tracts positioned farther away from the midline.
In AC or PC, the pan-neural expression of Fraextra-Robointra leads to a commissureless phenotype (Bashaw and Goodman 1999). Since the phenotype in this case appears to be similar to the commissureless (comm) mutant phenotype [where Robo is upregulated in the commissural tracts (Seeger et al. 1993; Tear et al. 1996)], Fraextra-Robointra must block not only the attraction mediated by Net-Fra but also any additional attractant signaling on commissural tracts mediated by some unknown players. It is also highly likely that the Robo-Sli direct repulsive signaling, unlike in the positioning of longitudinal tracts, significantly contributes to the guidance of commissural tracts (denoted by an R in Figure 7B) and, thus, the Fraextra-Robointra mimics a comm mutant phenotype.
Robo combinatorial code and Sli-Net interaction:
How does the Robo combinatorial code fit in with the Sli-Net results described in this article? Specifically, overexpression of Robo2 and Robo3, for example, drives the medial tracts more lateral (Rajagopalan et al. 2000; Simpson et al. 2000); how do we account for this result if the Sli signaling positions the lateral tracts via silencing of Net signaling? We believe that these results are consistent with the scenario in which the Sli-Robo interaction blocks events downstream of Fra. That is, a combination of signaling by Robo receptors interferes with the downstream events mediated by Fra. Driving Robo2 in medial tracts, for example, would interfere with the Fra signaling in such a way that the medial tract now adopts a more lateral tract trajectory. This is also consistent with the finding that the net phenotype is epistatic to the robo2 or robo3 phenotypes (data not shown). Therefore, both Robo2 and Robo3 must function via blocking the attraction by Net.
Acknowledgments
I thank Hugo Bellen, Steve Crews, Bassem Hassan, Roger Jacobs, Peter Kolodziej, John Nambu, Mark Seeger, Julie Simpson, Greg Bashaw, and Guy Tear for sharing various mutant stocks, antibodies, or information. I also thank Kai Zinn, Guy Benian, Barry Yedvobnick, Kevin Moses, Marc Tessier-Lavigne, and members of my lab for information, discussion, and comments. I thank Kathy Matthews at the Bloomington Stock Center for fly stocks and the Iowa Hybridoma Center for antibodies. This work is funded by the National Institutes of Health and the March of Dimes Foundation for Birth Defects.
References
- Ba-Charvet, N. K. T., K. Brose, V. Marillat, T. Kidd, C. S. Goodman et al., 1999. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron 22: 463–473. [DOI] [PubMed] [Google Scholar]
- Bashaw, G. J., and C. S. Goodman, 1999. Chimeric axon guidance receptors: the cytoplasmic domains of Slit and Netrin receptrs specify attraction versus repulsion. Cell 97: 917–926. [DOI] [PubMed] [Google Scholar]
- Bashaw, G. J., T. Kidd, D. Murray, T. Pawson and C. S. Goodman, 2000. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101: 703–715. [DOI] [PubMed] [Google Scholar]
- Bhat, K. M., 1996. The patched signaling pathway mediates repression of gooseberry allowing neuroblast specification by wingless during Drosophila neurogenesis. Development 122: 2921–2932. [DOI] [PubMed] [Google Scholar]
- Bhat, K. M., and P. Schedl, 1997. Requirement for engrailed and invected genes reveals novel regulatory interactions between engrailed/invected, patched, gooseberry and wingless during Drosophila neurogenesis. Development 124: 1675–1688. [DOI] [PubMed] [Google Scholar]
- Bhat, K. M., E. H. van Beers and P. Bhat, 2000. Sloppy paired acts as the downstream target of wingless in the Drosophila CNS and interaction between sloppy paired and gooseberry inhibits sloppy paired during neurogenesis. Development 127: 655–665. [DOI] [PubMed] [Google Scholar]
- Brose, K., K. S. Bland, K. H. Wang, D. Arnott, W. Henzel et al., 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 9: 795–806. [DOI] [PubMed] [Google Scholar]
- Chan, S. S., H. Zheng, M. W. Su, R. Wilk, M. T. Killeen et al., 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195. [DOI] [PubMed] [Google Scholar]
- Goodman, C. S., 1996. Mechanisms and molecules that control growth cone guidance. Annu. Rev. Neurosci. 19: 341–377. [DOI] [PubMed] [Google Scholar]
- Harris, R., L. M. Sabatelli and M. A. Seeger, 1996. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17: 217–228. [DOI] [PubMed] [Google Scholar]
- Harris, W. A., and C. E. Holt, 1999. Slit, the midline repellent. Nature 398: 462–463. [DOI] [PubMed] [Google Scholar]
- Hassan, B. A., N. A. Bermingham, Y. He, Y. Sun, Y. N. Jan et al., 2000. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron 25: 549–561. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., J. G. Culotti and D. H. Hall, 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85. [DOI] [PubMed] [Google Scholar]
- Ishii, N., W. G. Wadsworth, B. D. Stern, J. G. Culotti and E. M. Hedgecock, 1992. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9: 873–881. [DOI] [PubMed] [Google Scholar]
- Keleman, K., and B. J. Dickson, 2001. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32: 605–617. [DOI] [PubMed] [Google Scholar]
- Kidd, T., K. S. Bland and C. S. Goodman, 1999. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96: 785–794. [DOI] [PubMed] [Google Scholar]
- Kolodziej, P. A., L. C. Timpe, K. J. Mitchell, S. R. Fried, C. S. Goodman et al., 1996. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87: 197–204. [DOI] [PubMed] [Google Scholar]
- Li, H. S., J. H. Chen, W. Wu, T. Fagaly, T. L. Zhou et al., 1999. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96: 807–818. [DOI] [PubMed] [Google Scholar]
- Mitchell, K. J., J. L. Doyle, T. Serafini, T. E. Kennedy, M. Tessier-Lavigne et al., 1996. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17: 203–215. [DOI] [PubMed] [Google Scholar]
- Noordermeer, J., J. Klingensmith, N. Perrimon and R. Nusse, 1994. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 367: 80–83. [DOI] [PubMed] [Google Scholar]
- Rajagopalan, S., V. Vivancos, E. Nicolas and B. J. Dickson, 2000. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell 103: 1033–1045. [DOI] [PubMed] [Google Scholar]
- Ren, X. C., S. Kim, E. Fox, E. M. Hedgecock and W. G. Wadsworth, 1999. Role of netrin UNC-6 in patterning the longitudinal nerves of Caenorhabditis elegans. J. Neurobiol. 39: 107–118. [PubMed] [Google Scholar]
- Rothberg, J. M., J. R. Jacobs, C. S. Goodman and S. Artavanis-Tsakonas, 1990. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 4: 2169–2187. [DOI] [PubMed] [Google Scholar]
- Schindelholz, B., M. Knirr, R. Warrior and K. Zinn, 2001. Regulation of CNS and motor axon guidance in Drosophila by the receptor tyrosine phosphatase DPTP52F. Development 128: 4371–4378. [DOI] [PubMed] [Google Scholar]
- Seeger, M., G. Tear, M. D. Ferres and C. S. Goodman, 1993. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron 10: 409–426. [DOI] [PubMed] [Google Scholar]
- Seeger, M. A., and C. Beattie, 1999. Attraction versus repulsion: modular receptors make the difference in axon guidance. Cell 97: 821–824. [DOI] [PubMed] [Google Scholar]
- Siegfried, E., E. L. Wilder and N. Perrimon, 1994. Components of wingless signalling in Drosophila. Nature 367: 76–80. [DOI] [PubMed] [Google Scholar]
- Simpson, J. H., T. Kidd, K. S. Bland and C. S. Goodman, 2000. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron 28: 753–766. [DOI] [PubMed] [Google Scholar]
- Stein, E., and M. Tessier-Lavigne, 2001. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291: 1928–1938. [DOI] [PubMed] [Google Scholar]
- Tear, G., R. Harris, S. Sutaria, K. Kilomanski, C. S. Goodman et al., 1996. commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron 16: 501–514. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., 1994. Axon guidance by diffusible repellants and attractants. Curr. Opin. Genet. Dev. 4: 596–601. [DOI] [PubMed] [Google Scholar]
- Van Vactor, D., and J. G. Flanagan, 1999. The middle and the end: slit brings guidance and branching together in axon pathway selection. Neuron 2: 649–652. [DOI] [PubMed] [Google Scholar]
- Wang, K. H., K. Brose, D. Arnott, T. Kidd, C. S. Goodman et al., 1999. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 96: 771–784. [DOI] [PubMed] [Google Scholar]
- Wolf, B. D., and A. Chiba, 2000. Axon pathfinding proceeds normally despite disrupted growth cone decisions at CNS midline. Development 127: 2001–2009. [DOI] [PubMed] [Google Scholar]