Figure 2.
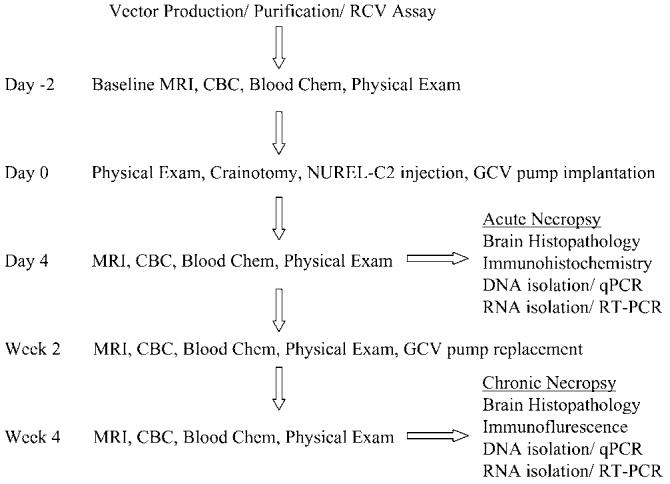
Flowchart of procedures performed. All animals were negative for Herpes-B on two independent tests prior to being enrolled in this study. Vector was produced and purified as described in Materials and methods and vector lots tested negative for RCV prior to use. At 2 days before surgery, the animals underwent a full examination consisting of a routine physical exam, MRI, CBC, and blood chemistry. Craniotomy, vector injection, and GCV pump implantation was performed as described in Materials and methods on day 0. At 4 days postinjection all animals received a second full standard examination. The acute panel of animals (4 days postinjection) was killed and necropsy processed for histopathology, immunohistochemistry, DNA, and RNA isolation. Both 2 and 4 weeks postinjection full examinations were again performed. Following the 4-week exam, the chronic animals were killed and necropsy processed as for acute animals.
