Figure 1.—
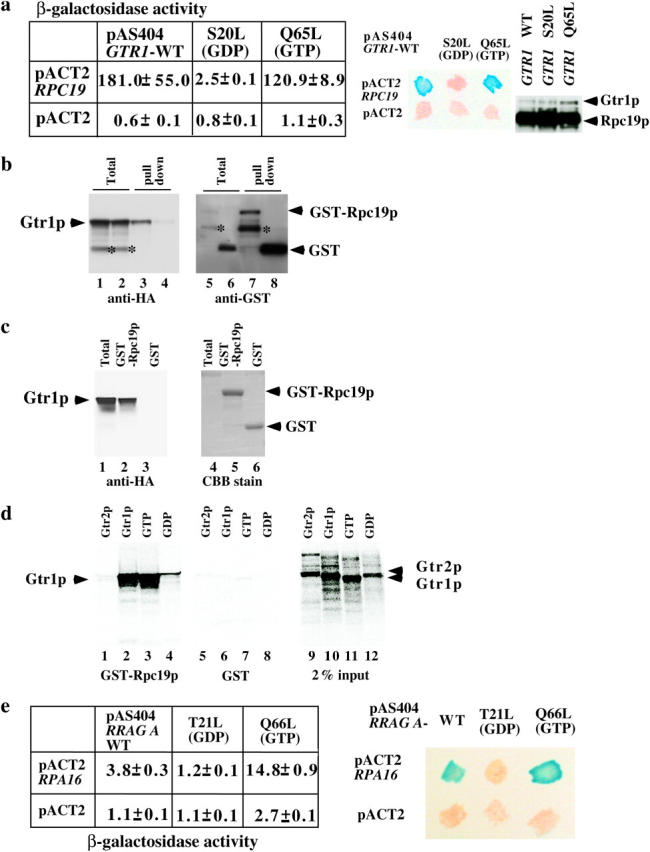
Association of Rpc19p with Gtr1p. (a) Extracts from cultures of S. cerevisiae colonies harboring two yeast genes, GTR1 wild type, S20L or the Q65L form in the pAS404 vector, or RPC19 in the pACT2 vector as shown, were obtained. Their β-galactosidase activities were measured as described before (Sekiguchi et al. 2001) and are shown as means of triplicate values with standard deviations. A β-galactosidase filter assay was also performed (middle). Patches of blue color appearing within 30 min represent a positive signal. White patches represent a negative signal. Expression of HA-tagged Gtr1p and Rpc19p in the yeast extracts described above was shown by immunoblotting against the HA tag (right). (b) Either GST-Rpc19p (lanes 1, 3, 5, and 7) or GST (lanes 2, 4, 6, and 8) was coexpressed with HA-tagged Gtr1p in NBW5 gtr1Δ. Prepared crude extracts were mixed with glutathione Sepharose-4B beads and pulled down as described previously (Nakashima et al. 1999). Bound proteins were run on SDS-polyacrylamide gels and transferred onto nylon membranes, which were immunoblotted with either anti-HA (lanes 1–4) or anti-GST (lanes 5–8) antibodies. Lanes 1, 2, 5, and 6: total proteins (10% input). An asterisk indicates degraded protein. (c) HA-tagged Gtr1p was expressed in NBW5 gtr1Δ. Yeast cell lysate was prepared and pulled down with either E. coli-produced GST-Rpc19p (lanes 2 and 5) or GST (lanes 3 and 6), which were bound to glutathione Sepharose-4B. Bound proteins were run on SDS-polyacrylamide gels and transferred onto nylon membranes, which were immunoblotted with the anti-HA (lanes 1–3) antibody. The filters were then stained with Coomassie Brilliant Blue R-250 (lanes 4–6). Lanes 1 and 4: total proteins (10% input). (d) 35S-labeled recombinant proteins (Gtr2p, lanes 1 and 5; Gtr1p, lanes 2 and 6; Gtr1p GTP form, lanes 3 and 7; the GDP form of Gtr1p, lanes 4 and 8) were produced with the TNT-Quick Coupled reaction kit and were pulled down with either GST-Rpc19p (lanes 1–4) or GST (lanes 5–8) protein, which was bound to glutathione Sepharose-4B beads. Bound proteins were run on SDS-polyacrylamide gels and analyzed using the Fuji image analyzer. Input proteins (2%) are shown in lanes 9–12. (e) Extracts from cultures of S. cerevisiae colonies harboring two human genes—RRAG A wild type (the T21L or the Q66L form in the pAS404 vector) and RPA16 in the pACT2 vector as shown—were obtained. Their β-galactosidase activities are shown as means of triplicate values with standard deviations. β-Galactosidase filter assay using Y190 strains harboring pAS404-RRAG A, pAS404-RRAG A(T21L) (GDP form), or pAS404-RRAG A(Q66L) (GTP form) and pACT2-RPA16 was performed as described above.
