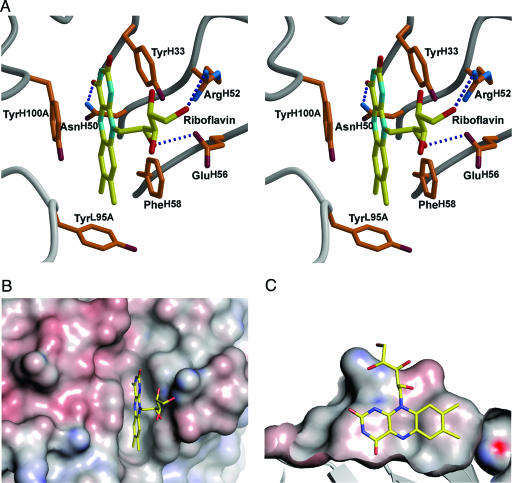
Fig. 3.
Stereoview of the IgGGAR antigen-binding site. (A) The combining site with bound riboflavin. Hydrogen bonds are shown as dotted blue lines. The isoalloxazine ring is π-stacked between aromatic residues TyrH33, PheH58, and TyrH100A, the N5 atom of the ring hydrogen bonds to AsnH50, and the ribityl side chain contributes two hydrogen bonds to ArgH52 and GluH56. (B and C) Top view (B) and side view (C) of shape complementarity of riboflavin in the antibody IgGGAR-combining site prepared with pymol (http://pymol.sourceforge.net). The isoalloxazine ring is trapped in a narrow slot in the combining site, and the ribityl side chain rests on the outer surface of the combining site. The molecular surface is colored by electrostatic potential (calculated with the program apbs (28) with a 1.4-Å probe radius and contoured between −20 and +20 kT).