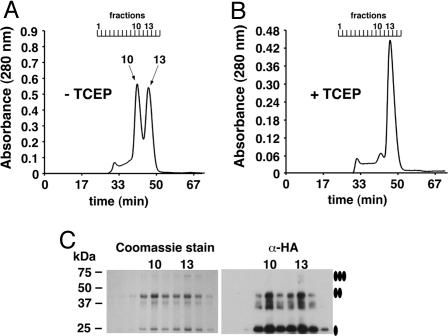
Fig. 2.
Gel filtration of purified HAhCTR1N15Q reveals two disulfide-dependent oligomerization states. (A) HAhCTR1N15Q in the absence of reducing agents. (B) HAhCTR1N15Q chromatographed after the column was preequilibrated with buffer containing the disulfide-specific reducing agent TCEP (1 mM). In the absence of TCEP, a HAhCTR1N15Q dimer-of-trimer species eluted in fraction 10 and the trimer eluted in fraction 13. (C) Coomassie staining of 10 μl each of gel filtration fractions 7–15 and the same fractions diluted 1:1,000 subjected to anti-HA Western blot. Fractions 10 and 13 are specifically indicated. The smear observed below the band of the dimer most likely reflects anomalous migration caused by flexibility of the dimeric species.