Abstract
We demonstrate that the susceptibility of human cancer cells to be infected and killed by an oncolytic poxvirus, myxoma virus (MV), is related to the basal level of endogenous phosphorylated Akt. We further demonstrate that nonpermissive tumor cells will switch from resistant to susceptible for MV infection after expression of ectopically active Akt (Myr-Akt) and that permissive cancer cells can be rendered nonpermissive by blocking Akt activation with a dominant-negative inhibitor of Akt. Finally, the activation of Akt by MV involves the formation of a complex between the viral host range ankyrin-repeat protein, M-T5, and Akt. We conclude that the Akt pathway is a key restriction determinant for permissiveness of human cancer cells by MV.
Keywords: oncolytic virus, poxvirus, virus tropism, PkB, M-T5
Myxoma virus (MV) is a rabbit specific poxvirus that causes a lethal disease called myxomatosis in European rabbits (Oryctolagus cuniculus) (1). MV encodes a wide complement of immune evasion molecules, including an ankyrin-repeat host range protein called M-T5 (2, 3). M-T5 acts to prevent apoptosis during infection of rabbit T lymphocytes and is a virulence factor for disease progression in infected rabbits (4). We have recently demonstrated that M-T5 also acts to protect MV-infected cells from cell cycle arrest through interactions with cullin-1, which is involved in regulation of p27 through ubiquitin-dependent proteasome degradation pathway (5). However, several aspects of M-T5 indicate that it exerts its host range functions in pathways above and beyond selective degradation. In particular, our group has recently shown MV exhibits potent oncolytic activity for human cancer and M-T5 is a critical determinant of MV tropism in human cancer cells (6, 7).
To investigate the tropism of MV in human tumor cells, we have examined cellular pathways of significance in human cancer. Akt, or protein kinase B (PKB), is a serine/threonine kinase that plays a central role in the regulation of cellular processes including proliferation, programmed cell death, angiogenesis, and metabolism (8). Akt is activated by a variety of stimuli, including growth factors, protein phosphatase inhibitors, and cellular stress in a phosphatidylinositol 3-kinase (PI3K)-dependent manner (9). The kinase activity of Akt contributes to the control of cell transformation and oncogenic activity and Akt activation is frequently dysregulated in human cancer cells (10, 11). Three mammalian Akt isoforms have been characterized and are identified as Akt1, Akt2, and Akt3. All three isoforms share the same structural organization with a conserved N terminus pleckstrin homology domain, a central kinase domain and a C-terminal regulatory domain (12). Akt1, normally called Akt, is the best characterized isoform and contains two phosphorylation sites, Thr-308 in the kinase domain and Ser-473 in the regulatory domain (13). However, phosphorylation of Thr-308 activates Akt, and full activation requires both Thr-308 and Ser-473 to be phosphorylated (14).
To further explain the mechanism of M-T5 action as an oncolytic virus host range factor in human cancers (6), here we report that MV infection requires Akt phosphorylation for productive infection of human cancer cells and this is mediated through interaction between M-T5 and Akt.
Results
Endogenous Levels of Phosphorylated Akt of Human Cancer Cells Are an Indicator of Susceptibility to MV Infection.
Human tumor cells were previously screened for either wild-type [vMyxlac (expressing β-gal) and vMyxgfp (expressing EGFP)] or a host range defective (vMyxT5KO) MV infection by our laboratory (6). We demonstrated two phenomena regarding MV infection of human cancer cells lines. First, human tumor cell lines exhibited one of three phenotypes after infection. Some tumor lines could support a productive infection by both wild-type or host range defective MV. A second group generated abortive, or nonproductive infection regardless of whether the infecting virus was wild type or the host range mutant. Finally, we observed a proportion of tumor lines that we described as restrictive. These cancer cells supported wild-type virus infection but were resistant to infection by the host range mutant. The second phenomenon we described was that expression of one specific MV host range gene, M-T5, is necessary for infection of restrictive cancer lines but dispensable for infection of supportive tumor cell lines (6). The cancer origin or tissue type of the tumor cell line could not explain this observation. However, when we surveyed possible oncogenic proteins that might differ among the lines we noticed that human cancer cell lines could be grouped into one of three types based on the levels of endogenous phosphorylated Akt, and that this grouping correlated with the permissiveness to MV infection (Table 1).
Table 1.
Akt activation correlates with permissiveness for MV
| Cell line | Cell origin | Endogenous |
p-Akt |
Permissive |
||||
|---|---|---|---|---|---|---|---|---|
| Akt | p-Akt | vMyxlac | vMyxT5KO | vMyxlac | vMyxT5KO | |||
| Controls | RK-13 | Kidney (rabbit) | + | High | + | + | + | + |
| BGMK | Kidney (primate) | + | High | + | + | + | + | |
| HEK293 | Kidney (human) | + | High | + | + | + | + | |
| Type I | HOS | Osteocarcoma | + | High | + | + | + | + |
| Caki-1 | Renal cancer | + | High | + | + | + | + | |
| PC3 | Prostate cancer | + | High | + | + | + | + | |
| Type II | HCT116 | Colon cancer | + | Low | + | − | + | − |
| 786–0 | Renal cancer | + | Low | + | − | + | − | |
| ACHN | Renal cancer | + | Low | + | − | + | − | |
| SK-OV-3 | Ovarian cancer | + | Low | + | − | + | − | |
| U373 | Glioma | + | Low | + | − | + | − | |
| Type III | MCF-7 | Breast cancer | + | None | − | − | − | − |
| COLO205 | Colon cancer | + | None | − | − | − | − | |
| MDA-MB435 | Breast cancer | + | None | − | − | − | − | |
| SK-MEL5 | Melanoma | + | None | − | − | − | − | |
+ indicates that endogenous Akt protein or phospho-Akt are detectable by immunoblotting or that cells were permissive to MV infection; − represents undetectable endogenous phosphorylated Akt Ser-473 or Thr-308, or that cells were nonpermissive to infection by MV. p-Akt, phospho-Akt.
All screened tumor lines expressed detectable levels of Akt but exhibited either high or low levels of endogenous phosphorylated Akt, or did not express any detectable phospho-Akt (Table 1). High levels of endogenous phospho-Akt were associated with type I cells, low or very low levels of phospho-Akt were detected in type II cells, and type III cells did not express any detectable endogenous phospho-Akt (Table 1 and Fig. 5A, which is published as supporting information on the PNAS web site). Significantly, this classification matched perfectly with the ability of MV or vMyxT5KO to infect each specific cell line. Type I cells were infected by either MV or vMyxT5KO, type II cells supported MV infection but were not permissive for vMyxT5KO infection, and type III cells did not support productive infection by either virus (Table 1).
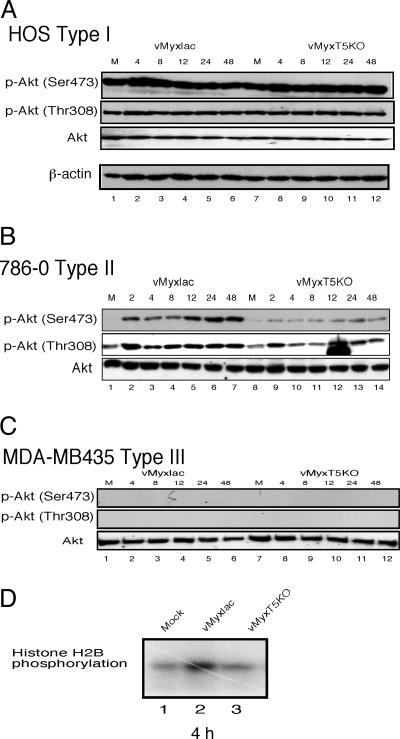
As an example of type I cells, endogenous phospho-Akt (both p-Akt Thr-308 and p-Akt Ser-473) is highly elevated in HOS cells (Fig. 1A, lanes 1 and 7) and infection with either vMyxlac or vMyxT5KO, both of which result in a productive infection (Fig. 5B), does not result in any further elevated levels of phophorylated Akt (Fig. 1A and Table 1). This finding suggests that certain cancer cell lines have sufficiently high levels of constitutively activated phospho-Akt at both Ser-473 and Thr-308 so as to support productive MV infection regardless of the expression of M-T5 and that infection does not induce an increase in the measurable levels of activated Akt.
Fig. 1.
Infection with wild-type MV, but not vMyxT5KO, dramatically induces phosphorylation level of Akt. HOS (human osteosarcoma) (A), human renal cancer 786-0 (B) and breast cancer MDA-MB435 (C) cells were mock-infected (M) or infected with either vMyxlac or vMyxT5KO at an MOI of 5. Human cancer cells were classified as type I (HOS), type II (786-0), or type III (MDA-MB435) based on the induction of phosphorylated Akt after MV infection. (D) In vitro Akt kinase assay. 786-0 cells were mock-infected (lane 1) or infected with either vMyxlac (lane 2) or vMyxT5KO (lane 3). Samples were collected at 4 h, and equal amounts of protein lysate (50 μg per reaction) were immunoprecipitated with anti-Akt antibody (2 μg per reaction), respectively. The immunoprecipitates were subjected to an in vitro kinase assay using histone H2B as the substrate.
In contrast, endogenous levels of Ser-473 phosphorylated Akt in 786-0 cells (type II) was very low, whereas p-Akt Thr-308 was detectably phosphorylated (Fig. 1B, lanes 1 and 8). However, infection of 786-0 cells with vMyxlac dramatically induced Akt phosphorylation at p-Akt Ser-473 and increased the level of P-Thr-308, but when 786-0 cells were infected with vMyxT5KO, the endogenous levels of phospho-Akt were relatively unchanged at both sites (Fig. 1B). These results were reproducible in other type II tumor cell lines, including HCT116, ACHN, U373, and SK-OV-3 (Table 1), but total Akt protein levels were unchanged in any cell after vMyxlac and vMyxT5KO infection (Fig. 1 and data not shown). This result suggests that M-T5 expression can activate Akt under conditions of MV infection in a cell line that is abortive for vMyxT5KO infection. Therefore, we postulated that activation of Akt is necessary to support productive MV infection in human tumor cell lines and that M-T5 has a role in activating Akt during MV infection (Fig. 5C). This finding is consistent with the observation that MV failed to infect type II cells, such as 786-0, in the absence of M-T5 expression (6).
Finally, infection of type III cells such as MDA-MB435 cells, a breast cancer cell line that is abortive for MV infection, exhibits undetectable endogenous phosphorylated Akt levels (Fig. 1C, lanes 1 and 7) and MV infection does not induce any measurable increased levels of activated Akt at either Ser-473 or Thr-308 (Fig. 1C). Nevertheless, the total Akt protein level was unchanged, similar to that observed for 786-0 and HOS cells. MDA-MB435 cells do not support productive infection by either vMyxlac or vMyxT5KO (Fig. 5D) (6), and we conclude that when expression of M-T5 is unable to induce the activation of Akt, the cancer cells remains nonpermissive (Table 1). Thus, the level of phosphorylated Akt, whether endogenous or virus-induced, is predictive for which cancer cell lines will support productive MV infection (Table 1).
To confirm that MV infection induced the activation of Akt kinase activity in permissive cells, we performed an in vitro kinase assay. Type II 786-0 cells were mock-infected or infected with either vMyxlac or vMyxT5KO, collected at 4 h postinfection (hpi), and immunoprecipitated with anti-Akt antibody. The immunoprecipitates were subjected to an in vitro kinase assay using histone H2B as the substrate (Fig. 1D). This result indicates that Akt kinase activity can be increased in 786-0 cells by infection with vMyxlac, but not with vMyxT5KO, which is consistent with immunoblotting analysis in the same cell line. This result is reproducible in other type II cells such as ACHN, SK-OV3, and U373 (data not shown). Taken together, these data indicate that phosphorylation of Akt is predictive for productive MV infection in specific human cancer cells.
Akt Phosphorylation Is Resistant to PI3K/Akt Drug Inhibitors After MV Infection.
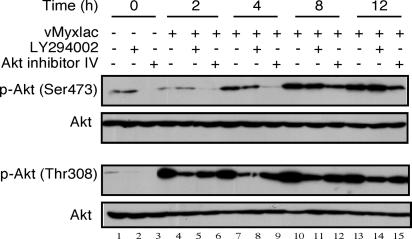
Type II cells exhibited the largest change in activated levels of Akt after MV infection (see Fig. 1B); therefore, we used the human renal cancer cells 786-0 as our representative type II cell to test whether drugs that inhibit PI3K/Akt could block Akt phosphorylation activated by infection of MV. LY294002 is a potent and specific inhibitor of PI3K and blocks downstream pathways of PI3K, including Akt activation (15, 16). In contrast, Akt inhibitor IV was designed to target an ATP-binding site of a kinase immediately upstream of Akt, but downstream of PI3K, thereby specifically inhibiting Akt phosphorylation (17). The most likely candidate for inhibition is PDK1; however, this remains to be confirmed conclusively. Human renal cancer (786-0) cells were pretreated with LY294002 (50 μM) or Akt inhibitor IV (10 μM) for 1 h and then infected with vMyxlac (multiplicity of infection, MOI, of 5). At various times after infection, vMyxlac-infected cells were collected and whole cell lysates were prepared. The results show that treatment of uninfected 786-0 cells with LY294002 or Akt inhibitor IV blocked phosphorylation of Akt (Fig. 2, lanes 1–3). However, treatment with the inhibitors did not appear to significantly inhibit the increased phosphorylation of Akt at Ser-473 or Thr-308 after MV infection (Fig. 2). There was some reduction early in the infection (0–4 hpi) when compared to untreated control samples (Fig. 2 Upper). This finding suggests that activation of Akt depends on MV infection and under the control of the expression of M-T5 protein, and when either PI3K or downstream activation of Akt are blocked by LY294002 or Akt inhibitor IV (Fig. 2 Lower), then wild-type MV is still robustly capable of activating Akt. Therefore, we conclude that specific Akt inhibitors are unable to significantly block Akt activation after infection by vMyxlac in time-dependent manner, and that M-T5 acts downstream of these inhibitors.
Fig. 2.
The phosphorylation of Akt induced by MV infection is insensitive to PI3K/Akt inhibitors in a time-dependent manner. Human renal cancer cells (786-0) were pretreated with PI3K or Akt inhibitors (50 μM LY294002 or 10 μM AKT inhibitor IV) for 1 h, and then the cells were infected with vMyxlac (MOI of 5). Akt phosphorylated at both p-Akt Ser-473 and p-Akt Thr-308 sites were detected in cell lysates (50 μg per lane) by using immunoblotting at various time points (0–12 hpi). Total Akt levels were not changed and taken as a loading control.
Inhibition of the PI3K pathway with LY294002 or Akt inhibitor IV did not induce cell death as measured by trypan blue staining when type II were infected with vMyxlac (data not shown). In particular, when we tested levels of phospho-Bad, caspase 3, and poly(ADP ribose polymerase) (PARP), they were also unchanged (data not shown). Together with data shown in Fig. 1, we propose that M-T5 plays a direct role in activation of Akt phosphorylation after infection in type II cells. We then tested whether M-T5 activates Akt through protein–protein interaction.
M-T5 Interacts with Akt Under both Transfection and Virus Infection Conditions.
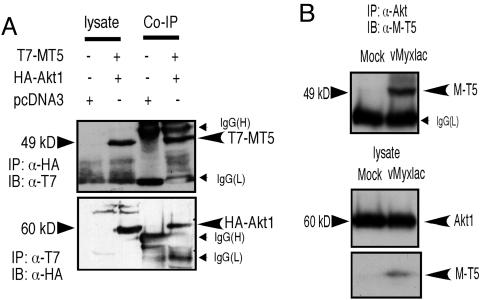
To confirm any physical association between M-T5 and Akt, HEK293 cells were cotransfected with tagged M-T5 (pcDNA3T7-MT5) and tagged Akt (pcDNA3HA-Akt1). Twenty-four hours after transfection, immunocomplexes were precipitated with anti-T7 to pull down T7-MT5 fusions, and any complexes were immunoblotted with anti-hemagglutinin (HA) antibody. When reciprocal immunoprecipitations were performed, T7 tagged M-T5 was detected in the HA-Akt immunoprecipitates (Fig. 3A Upper), and HA-Akt was coimmunoprecipitated by anti-T7 antibody (Fig. 3A Lower). These reciprocal coimmunoprecipitations confirmed that M-T5 forms complexes with Akt (Fig. 3). To confirm the ability of M-T5 to also interact with endogenous untagged human Akt after MV infection, we examined type II 786-0 cells infected with vMyxlac at an MOI of 5. Twelve hpi, the cells were collected and lysed, and immune complexes were precipitated with an anti-Akt antibody. Immunoblotting with anti-T5 antiserum indicated that expression of T5 protein was able to coprecipitate with endogenous Akt from MV-infected human cancer cells in vivo (Fig. 3B Upper).
Fig. 3.
M-T5 interacts with Akt under both transfection and infection conditions. (A) HEK293 cells were cotransfected with pcDNA3T7-MT5 and either pcDNA3HA-Akt1 or an empty vector for 24 h. Coimmunoprecipitations (Co-IP) were performed in kinase lysis buffer using anti-HA (12CA5) or anti-T7 to precipitate complexes. Immunoblot (IB) analysis in SDS/8% PAGE detected the resulting immune complexes with anti-T7 (Upper) or anti-HA (Lower), respectively. Expression of the transfected plasmids is shown in the left lane. (B) M-T5 interacts with endogenous Akt in 786-0 cells after MV infection. 786-0 cells were infected with vMyxlac (MOI of 5) and, at 12 h after infection, immune complexes were pulled down with anti-Akt and immunoblotted with anti-MT5 (Upper). Expression of M-T5 and endogenous human AKT is indicated (Lower).
Also, additional coimmunoprecipitations from cells transfected with plasmids that express both M-T5 and HA-tagged PI3K, including both subunits (p110 and p85) or HA-Bad, as well as by virus infected cell coimmunoprecipitation, confirmed that M-T5 was unable to bind to exogenous or endogenous p85 or p110 or the downstream regulator Bad under either transfection or infection conditions (data not shown). Therefore, these data suggest that M-T5 is able to specifically interact with Akt and that this interaction is a requirement for enhancement of Akt activation.
Activation of Akt Is Required for MV Infection.
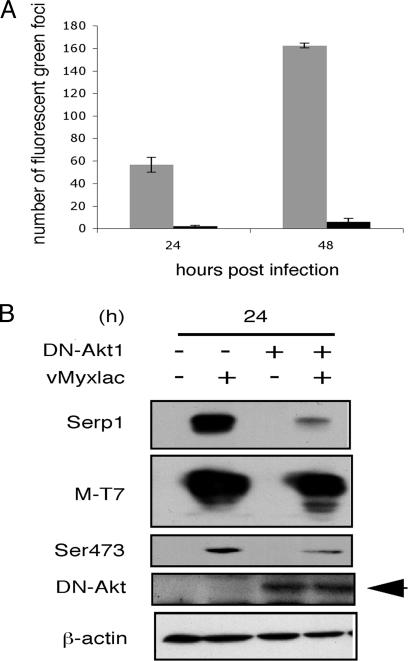
As shown in Fig. 1, Akt becomes activated after vMyxlac infection in type II cells. We have also demonstrated that the Akt specific inhibitor IV, which acts immediately upstream of Akt, does not block Akt activation after MV infection. Therefore, we transfected of 786-0 cells with a dominant negative (DN) Akt cassette (HA-DN-Akt1) for 12 h and then infected with MV (vMyxgfp) at three MOIs to measure any inhibitory effect of Akt activation by DN-Akt expression upon MV infection. We observed that DN-Akt can dramatically inhibit MV infection of 786-0 cells (Fig. 4and Fig. 6, which is published as supporting information on the PNAS web site). Transfection of DN-Akt followed by infection of vMyxgfp caused a significant decrease in the number of gfp-expressing cell numbers at 24 and 48 h at various MOIs (Figs. 4A and 6A). 786-0 cells transfected with DN-Akt and then infected with vMyxgfp for 24 or 48 h (MOI of 1) produced significantly fewer foci than the control transfections/infections (Fig. 4A) and reduced viral titres (Fig. 6B). We obtained similar results with other type II cells (ACHN and SK-OV3) that were transfected with DN-Akt and then infected with MV (data not shown). These data demonstrate that inhibition of Akt signaling interferes with MV infection and spread. Therefore, we conclude that Akt is a determinant of productive MV infection in type II human cancer cells and likely also explains the M-T5 independence of MV replication in type I cells, in which Akt is already constitutively activated. However, if Akt activation is blocked in type I cells by transfection of DN-Akt, then MV replication is also reduced, thus confirming that activated Akt is necessary for productive MV infection (Fig. 6C).
Fig. 4.
Activation of Akt is required for virus infection and the formation of foci in MV. Human renal cancer cells (786-0) were transfected with an HA-tagged DN-Akt plasmid (black) or pcDNA3 vector alone (gray) for 12 h and then infected with MV (vMyxGFP) at MOIs of 0.01, 0.1, and 1 for 24 (not shown) or 48 (A) h, respectively. (A) Fluorescent foci were enumerated under fluorescent microscopy. (B) Inhibition of Akt signaling significantly reduces late viral gene expression. 786-0 cells were transfected with DN-Akt for 12 h and then infected with vMyxGFP at an MOI of 1 for 24 h. The expression of DN-Akt results in a subsequent decrease in the expression of the late viral gene Serp1 and levels of p-Akt Ser-473, but the early gene T7 expression is not affected. Actin was used as a loading control.
Inhibition of Akt Signaling Significantly Reduces MV Replication at the Level of Late Viral Gene Expression.
To further confirm the influence of direct inhibition of Akt on MV replication, we assessed the expression levels of representative MV early (M-T7) and late (Serp1) viral genes after infection of 786-0 cells with vMyxgfp (MOI of 1). The efficiency of transfection for 786-0 cells was 90–95%, which was monitored by GFP vector transfection (data not shown). 786-0 cells were transfected with DN-Akt or control vector for 12 h and then infected with vMyxgfp. Transfection of DN-Akt did not affect the expression of the early gene, M-T7; however, there was a significant decrease in expression of the viral late gene, Serp1 (Fig. 4B). These data indicate that inhibition of Akt activation has little affect on early viral gene expression but reduced late viral gene expression, suggesting that inhibition of Akt signaling resulted in a late stage block to MV replication. This observation is consistent with the data obtained in Figs. 4A and 6 and, taken together, indicate that activation of Akt is essential for completing the full MV replication cycle and that M-T5 is critical through its interaction with Akt. These findings were also reproduced in other type II cells (ACHN and SK-OV3; data not shown). We conclude that, if Akt activation is blocked or M-T5 expression is ablated, then MV cannot productively infect type II cancer cells.
Transient Expression of Constitutively Active Akt1 Facilitates MV Infection of Nonpermissive Cancer Cells.
It is curious why wild-type MV is unable to induce activation of Akt after infection of type III cells. A cellular block to virus entry and early gene expression might explain the observed failure to replicate. Alternatively, a dysregulation of Akt activation by M-T5 might also explain this apparent abort of MV infection of type III cells. To test these alternative explanations, we infected each cell type with vMyxlac and then assessed viral gene expression by immunofluorescence (Fig. 7, which is published as supporting information on the PNAS web site). Type I and II cells exhibited similar patterns of punctate cytoplasmic M-T5 staining. However, there was either decreased M-T5 expression or stability, or possibly aberrant localization in the type III cells, despite the fact that a control early viral protein (M-T7) was expressed normally. This finding suggested that the failure of MV infection in type III was not due to a block to virus entry or early gene expression. We next reasoned that if phosphorylation of Akt was necessary for MV replication in cells that exhibit very low activated Akt levels (type II cells, Table 1), then expression of a constitutively active Akt cassette (HA-Myr-Akt) in cells that are nonpermissive to infection, and do not exhibit detectable levels of endogenous phosphorylated Akt levels (i.e., type III cells), might convert them from nonpermissive to permissive for MV infection. We selected the highly transfectable human breast cancer cells MDA-MB435, as an example of nonpermissive type III cells (Table 1) to test our hypothesis that constitutive expression of activated Akt could rescue the ability of MV to infect resistant cancer cell lines. A constitutively active Akt expression construct (HA-Myr-Akt1) or control vector (pcDNA3) were transfected into MDA-MB435 cells, and 12 hpi they were infected with vMyxgfp at an MOI of 0.01, 0.1, or 1.0. We observed classic MV foci expressing GFP at 48 hpi from cells expressing Myr-Akt, which were not detected in control cells that were infected only with MV (Fig. 8A, which is published as supporting information on the PNAS web site). Foci were enumerated under the florescent microscope and the results indicated that transfection of activated Akt followed by infection with MV dramatically increased levels of MV replication (Fig. 8B). Similar results were also obtained with another noninfectable type III cancer line (MCF-7; data not shown). These data indicate that the expression of constitutively active Akt is able to rescue MV infectivity in type III nonpermissive human cancer cells. In contrast, when we transfected M-T5 into type III cells, we could not rescue MV infection (Fig. 8C), suggesting that the block to a productive infection lies in either the inability of M-T5 to bind and activate Akt, or a dysregulation of the Akt signaling pathway itself. Taken together, our results indicate that activated Akt is necessary for MV infection of human cancer cells and nonpermissive cells can be rendered permissive by the ectopic expression of constitutively active Akt.
Discussion
A number of viruses have developed strategies to survive for long periods in the host and the PI3K-Akt signaling pathway, in particular, has attracted much interest due to its central role in the regulation of apoptotic inhibition (18). Activation of the PI3K-Akt signaling pathway is believed to contribute to increased cell survival, and seems to be a common strategy for many viruses to manipulate cell survival pathways until the virus life cycle is complete (19). Some viruses inhibit apoptosis, and others accelerate it, depending on the biological strategy of that particular virus (20, 21). Infection with some viruses, such as respiratory syncytial virus (RSV), immediately induces activation of Akt through PI3K-NFkB mediated antiapoptotic signaling at a very early stage of infection (22). In contrast, other viruses, such as hepatitis B, hepatitis C, and flaviviruses, encode gene products that directly induce PI3K-Akt phosphorylation which contributes to an antiapoptosis strategy (23–25). In this study, we treated several human cancer cells that exhibit activated Akt with LY294002 and AKT inhibitor IV, and after MV infection, no apoptosis was detected with or without the inhibitors (data not shown). This finding demonstrates MV infection induced activation of Akt in a manner independent of PI3K activation and was resistant to inhibitors upstream of Akt in a time-dependent manner (Fig. 2). Also, inhibition of Akt activation by expression of DN-Akt effectively prevented productive MV infection. In contrast, human cytomegalovirus (HCMV) induces PI3K activation throughout the course of infection of quiescent fibroblasts (26). HCMV infection caused immediate PI3K activation at 15–30 min after infection, and PI3K inhibitor could thus inhibit virus replication (26).
The M-T5 host range factor of MV does not share significant similarity with any other nonviral proteins, although it is related to other poxvirus host range proteins, many of which have been defined by their ability to mediate viral tropism (27). M-T5 possesses seven ankyrin-repeat domains within the N-terminal and central regions of the protein (6). The ankyrin repeat, a 33-residue sequence motif, has been found in many proteins from viruses, bacteria, and eukaryotes, are thought to mediate specific protein–protein interactions (28). We recently identified the E3 ligase complex member cullin-1 as a binding partner of M-T5 and demonstrated that the M-T5/cul1 complex functions to regulate cell cycle through p27 degradation pathway in MV-infected cells including human tumor cells (5). Here we identify cellular Akt as a second, additional binding partner of M-T5 that regulates Akt signaling and is an important tropism factor for MV infection in human cancer type II cells. In the present study, we were unable to detect any M-T5 binding to either the p85 or p110 subunits of PI3K (data not shown). Because the induction of Akt by M-T5 is insensitive to PI3K/Akt inhibitors (Fig. 2), we postulate that M-T5 operates directly in conjunction with Akt. Experiments are underway to explore the role of downstream targets of Akt. Although we cannot yet deduce whether M-T5 alters availability of Akt to kinases or phosphatases, we do note that the activation of Ser-473 in the presence of M-T5 is particularly dramatic (Fig. 1B). Thus, M-T5 is a good candidate to regulate Akt activation via either the Akt kinase, mTOR/Rictor:GβL, or the Akt phosphatase, PHLPP (29, 30).
MV has been shown to exhibit tropism for many human cancer cells (6) and to be a potent oncolytic therapeutic for human gliomas in a murine xenograft model (7). The results in this study suggest that Akt manipulation may allow the oncolytic capacity of this virus to extend to an even broader spectrum of human cancer cells.
Materials and Methods
Cell Culture.
Established cell lines used in this study included rabbit kidney (RK13), baby green monkey kidney (BGMK), and human embryonic kidney (HEK) 293 epithelial cells. Human cancer cells including human osteosarcoma (HOS) cells, glioma (U373) cells, human renal cancer lines (786-0, Caki-1, ACHN), prostate cancer (DU145, PC3) cells, breast cancer (MDA-MB435, MCF-7, T47D) cells, colon cancer (HCT116, COLO205) cells, ovarian cancer (SK-OV3, OVCAR5) cells, and melanoma (SK-MEL5) cells were obtained from the NCI-60 reference collection received from J. Bell (Ottawa Cancer Institute, Ottawa). All cells were grown in DMEM supplemented with 10% FBS (Sigma), 100 units of penicillin per ml, and 100 μg of streptomycin per ml (Invitrogen).
Viruses and Infections.
The viruses used in this study, including vMyxlac (31), vMyxT5KO (4), and vMyxgfp (32), have been described. All viruses were propagated and titrated by focus formation on BGMK cells as described (31).
Antibodies and Reagents.
Generation of polyclonal anti M-T5 antiserum, mouse monoclonal antibody of anti-Serp1, and rabbit polyclonal antibody of T-7 have been described (5, 33, 34). Rabbit polyclonal phospho-Akt (Thr-308), mouse monoclonal phospho-Akt (Ser-473) (587F11) antibody, and polyclonal Akt antibody that detects total levels of endogenous Akt1, Akt2, and Akt3 proteins were obtained from Cell Signaling Technology. Mouse monoclonal antibody against the epitope of HA protein of human influenza virus (clone 12CA5) (Roche) and a peptide epitope directed against the 11-aa gene (T7-Tag; Novagen), a goat polyclonal antibody directed against the C terminus of actin (C-11) of human origin (Santa Cruz Biotechnology), and horseradish peroxidase-coupled goat anti-mouse or goat anti-rabbit secondary antibodies were obtained from Jackson ImmunoResearch. PI3K-AKT kinase inhibitors (LY294002 and AKT inhibitor IV) and [γ-32P]ATP were purchased from Calbiochem and PerkinElmer Life Sciences, respectively. Histone H2B was used as exogenous substrate (Roche Applied Science).
Plasmids.
M-T5 was amplified from MV genomic DNA (3) by PCR using primers that incorporated T7 Tag sequences into the expression vector pcDNA3 (pcDNA3T7-MT5) (5). The CMV-based expression constructs encoding wild-type HA-Akt1, constitutively active HA-Myr-Akt1 have been described (35, 36). Dominant negative, kinase-dead, mutant HA-DN-Akt1 cassette was prepared by point mutation method. HA-tagged PI3K, including both subunits (p110 and p85), have been described (37).
Cellular Bad was amplified by RT-PCR from HEK293 cells and cloned into HA-pcDNA3 vector (HA-Bad).
Immunoprecipation and Immunoblotting.
HEK293 cells were cotransfected by the calcium phosphate method (Clontech) for 24 h with plasmids HA-Akt1, HA-tagged PI3K, including both subunits (p110 and p85), and HA-Bad together with pcDNA3T7-MT5 or the vector alone, respectively. Immunoprecipitations were performed as described (38). Immunocomplexes were resolved by SDS/PAGE and then analyzed by Western blotting with appropriate antibodies. Immunoreactive proteins were detected by chemiluminescence (PerkinElmer). Loading of equal amounts of protein (50 μg per lane) from each sample was confirmed by detection of the housekeeping gene actin.
Transfection, PI3K, and Akt Kinase Inhibitor Treatments.
Cells were seeded in six-well plates at a density of 5 × 105 cells per well in complete growth medium with 10% FBS. Transfections were performed with LipofectAMINE 2000 (Invitrogen) in accordance with the manufacturer’s instructions. 786-0 or MDA-MB435 cells were transfected with HA-DN-Akt1, HA-Myr-Akt1 plasmid, or pcDNA3 alone (4 μg). Transfection efficiency was determined by expression of a GFP vector and found to be 90–95% efficient. For inhibition experiments, cells were serum-starved overnight and treated with PI3K and Akt kinase inhibitors LY29004 (50 μM) or Akt kinase IV (10 μM) for 1 h, then infected with vMyxlac (MOI of 5) for 1 h. After removal of the inoculum, the same inhibitor was added to cells and grown in complete growth medium supplemented with 10% FBS. The cells were collected at various time points. The lysate was used for detection with appropriate antibodies.
In Vitro Kinase Assay.
Protein kinase assays were performed as described (39). The proteins were separated on SDS/PAGE gels. Each experiment was repeated three times, and the relative amounts of incorporated radioactivity were determined by autoradiography.
Measurements of Akt Inhibition or Activation After Virus Infection.
Human renal cancer cells (786-0) were transfected with HA-DN-Akt or the empty vector pcDNA3 vector for 12 h. Cells were then infected with vMyxgfp virus for 24 and 48 h at several MOIs. The infected supernatants were used for detection of the expression of M-T7 (early MV protein) and Serp1 (late MV protein) by immunoblotting. The fluorescent foci were enumerated under fluorescent microscopy at 24 and 48 hpi. Cells were harvested and fixed with 1% formaldehyde, and fluorescent green cell numbers were counted by flow cytometry. To measure the effect of activation of Akt kinase on virus infection, MDA-MB435 cells were transfected with constitutively active Akt1 (HA-Myr-Akt) (4 μg) or vector alone for 12 h and then infected with vMyxgfp for 24, 48, and 72 h at MOIs of 0.01, 0.1, and 1, respectively. Foci numbers were counted under a Leica fluorescent microscope as a measure of virus spread.
Supplementary Material
Acknowledgments
We thank Z. Yuan (Harvard Medical School, Boston) for invaluable discussions and suggestions. This work was supported by National Cancer Institute of Canada and Canadian Institutes of Health Research grants (to G.M.). G.M. holds a Canada Research Chair in Molecular Virology and is an International Scholar of The Howard Hughes Medical Institute.
Abbreviations
- MV
myxoma virus
- PI3K
phosphatidylinositol 3-kinase
- hpi
hours postinfection
- MOI
multiplicity of infection
- HA
hemagglutinin
- DN
dominant negative.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fenner F., Ratcliffe F. N. Myxomatosis. Cambridge, U.K.: Cambridge Univ. Press; 1965. [Google Scholar]
- 2.Kerr P., McFadden G. Viral Immunol. 2002;15:229–246. doi: 10.1089/08828240260066198. [DOI] [PubMed] [Google Scholar]
- 3.Cameron C., Hota-Mitchell S., Chen L., Barrett J., Cao J.-X., Macaulay C., Willer D., Evans D., McFadden G. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. [DOI] [PubMed] [Google Scholar]
- 4.Mossman K., Lee S. F., Barry M., Boshkov L., McFadden G. J. Virol. 1996;70:4394–4410. doi: 10.1128/jvi.70.7.4394-4410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston J. B., Wang G., Barrett J. W., Nazarian S. H., Colvill K., Moran M., McFadden G. J. Virol. 2005;79:10750–70763. doi: 10.1128/JVI.79.16.10750-10763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sypula J., Wang F., Ma Y., Bell J., McFadden G. Gene Ther. Mol. Biol. 2004;8:103–114. [Google Scholar]
- 7.Lun X., Yang W., Alain T., Shi Z.-Q., Muzik H., Barrett J. W., McFadden G., Bell J., Hamilton M. G., Senger D. L., Forsyth P. A. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazil D. P., Yang Z. Z., Hemmings B. A. Trends Biochem. Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Foulstone E., Prince S., Zaccheo O., Burns J. L., Harper J., Jacobs C., Church D., Hassan A. B. J. Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- 10.Testa J. R., Bellacosa A. Proc. Natl. Acad. Sci. USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson K. M., Anderson N. G. Cell. Signaling. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 12.Song G., Ouyang G., Bao S. J. Cell Mol. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta S. R., Brunet A., Greenberg M. E. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 14.Bayascas J. R., Alessi D. R. Mol. Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Wennstrom S., Downward J. Mol. Cell. Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King W. G., Mattaliano M. D., Chan T. O., Tsichlis P. N., Brugge J. S. Mol. Cell. Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kau T. R., Schroeder F., Ramaswamy S., Wojciechowski C. L., Zhao J. J., Roberts T. M., Clardy J., Sellers W. R., Silver P. A. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor M. A., Alessi D. R. J. Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 19.Cooray S. J. Gen. Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 20.Roulston A., Marcellus R. C., Branton P. E. Annu. Rev. Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 21.Benedict C. A., Norris P. S., Ware C. F. Nat. Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 22.Thomas K. W., Monick M. M., Staber J. M., Yarovinsky T., Carter A. B., Hunninghake G. W. J. Biol. Chem. 2002;277:492–501. doi: 10.1074/jbc.M108107200. [DOI] [PubMed] [Google Scholar]
- 23.Lan K. H., Sheu M. L., Hwang S. J., Yen S. H., Chen S. Y., Wu J. C., Wang Y. J., Kato N., Omata M., Chang F. Y., Lee S. D. Oncogene. 2002;21:4801–4811. doi: 10.1038/sj.onc.1205589. [DOI] [PubMed] [Google Scholar]
- 24.Mannova P., Beretta L. J. Virol. 2005;79:8742–8749. doi: 10.1128/JVI.79.14.8742-8749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C. J., Liao C. L., Lin Y. L. J. Virol. 2005;79:8388–8399. doi: 10.1128/JVI.79.13.8388-8399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R. A., Wang X., Ma X. L., Huong S. M., Huang E. S. J. Virol. 2001;75:6022–6032. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden G. Nat. Rev. Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosavi L. K., Cammett T. J., Desrosiers D. C., Peng Z.-Y. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Gao T., Furnari F., Newton A. C. Mol. Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Opgenorth A., Graham K., Nation N., Strayer D., McFadden G. J. Virol. 1992;66:4720–4731. doi: 10.1128/jvi.66.8.4720-4731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston J. B., Barrett J. W., Chang W., Chung C. S., Zeng W., Masters J., Mann M., Wang F., Cao J., McFadden G. J. Virol. 2003;77:5877–5888. doi: 10.1128/JVI.77.10.5877-5888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nash P., Whitty A., Handwerker J., Macen J., McFadden G. J. Biol. Chem. 1998;273:20982–20991. doi: 10.1074/jbc.273.33.20982. [DOI] [PubMed] [Google Scholar]
- 34.Lalani A. S., Graham K., Mossman K., Rajarathnam K., Clark-Lewis I., Kelvin D., McFadden G. J. Virol. 1997;71:4356–4363. doi: 10.1128/jvi.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang K., Coppola D., Crespo N. C., Nicosia S. V., Hamilton A. D., Sebti S. M., Cheng J. Q. Mol. Cell. Biol. 2000;20:139–148. doi: 10.1128/mcb.20.1.139-148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 37.Sun M., Yang L., Feldman R. I., Sun X.-m., Bhalla K. N., Jove R., Nicosia S. V., Cheng J. Q. J. Biol. Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 38.Su J., Wang G., Barrett J. W., Irvine T. S., Gao X., McFadden G. J. Virol. 2006;80:1140–1151. doi: 10.1128/JVI.80.3.1140-1151.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Z. Q., Sun M., Feldman R. I., Wang G., Ma X., Jiang C., Coppola D., Nicosia S. V., Cheng J. Q. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.