Figure 4.
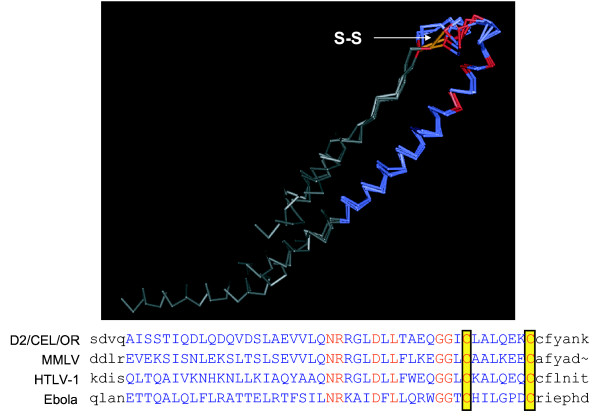
Structural and sequence alignment of the highly conserved putative disulfide-bonded loop region of the SRV-2 env and structurally similar regions of other viral envelope proteins. Structural similarities to the SRV-2 env protein were identified by querying the NCBI protein structure database using 3D-PSSM and Cn3D. A region within the C-terminal domain of SRV-2 env which was identical in all SRV-2 isolates and SRV-1 and SRV-3 prototypes (aa426-471) was predicted to have structural similarities to a disulfide-bonded loop presumed to be important for virus-cell fusion in a number of RNA viruses and retroviruses, including Ebola virus 1: 1EBO_A (Gp2); Ebola virus 2: 2EBO_A (Gp2); MMLV (Moloney murine leukemia virus): 1MOF (coat protein); HTLV-1 (human T-lymphotropic virus): 1MG1_A (gp21) (see text). The disulfide bridge is indicated by S-S.
