Abstract
Sphingomyelin (SM), the second most abundant phospholipid in plasma lipoproteins, was previously shown to be a physiological inhibitor of the lecithin-cholesterol acyltransferase (LCAT) reaction. In this study, we investigated the effects of its metabolites, ceramide and ceramide phosphate, on the activity and fatty acid specificity of LCAT in vitro. Treatment of SM-containing substrate with SMase C, which hydrolyzes SM to ceramide, abolished the inhibitory effect of SM, whereas treatment with SMase D, which hydrolyzes it to ceramide phosphate, increased the inhibition. Although incorporation of ceramide into the substrate in the absence of SM activated the LCAT reaction only modestly, its co-incorporation with SM neutralized the inhibitory effect of SM. Ceramide phosphate, on the other hand, inhibited the LCAT reaction more strongly than SM. The effects of the sphingolipids were similar on the phospholipase A and cholesterol esterification reactions of the enzyme, indicating that they regulate the binding of phosphatidylcholine (PC) to the active site, rather than the esterification step. Ceramide incorporation into the substrate stimulated the synthesis of unsaturated cholesteryl esters at the expense of saturated esters. However these effects on fatty acid specificity disappeared when the PC substrates were incorporated into an inert diether PC matrix, suggesting that ceramide increases the availability of polyunsaturated PCs to the enzyme by altering the macromolecular structure of the substrate particle. Since the plasma ceramide levels are increased during inflammation, these results indicate that the activity and fatty acid specificity of LCAT may be altered during the inflammatory response.
Abbreviations used: Apo : apolipoprotein, CE: cholesteryl ester, FC: free (unesterified) cholesterol, HDL: high density lipoproteins, LCAT: lecithin-cholesterol acyltransferase, LDL: low density lipoproteins, PC: phosphatidylcholine, PLA: phospholipase A, SM: sphingomyelin, SMase: sphingomyelinase
Sphingomyelin (SM) is one of the most abundant phospholipids in cell membranes and lipoproteins, constituting up to 30% of certain lipoprotein fractions (1,2). Although its role as a structural component of membranes, and as a precursor of signaling molecules has been well recognized, its function in plasma lipoproteins has received less attention. Recent studies show that plasma SM may be an independent risk factor for atherosclerosis (3), and that a reduction in SM levels by myriocin treatment reduces atherosclerosis in apo E deficient mice (4,5). While the exact mechanism of the proatherogenic effect of SM is unknown, one proposed mechanism involves the generation of ceramide in LDL by the action of arterial SMase, which causes increased retention, aggregation and oxidation of LDL, with subsequent uptake by the scavenger receptors of the macrophage (3). The formation of large amounts of ceramide in the plasma compartment is unlikely because of the absence of an active SMase in normal plasma. However, under inflammatory conditions, significant amounts of SMase may be released into circulation and cause the hydrolysis of lipoprotein SM to ceramide (6). Furthermore, de novosynthesized ceramide is secreted with the newly assembled lipoproteins by the liver during inflammatory reactions (7,8), and the presence of an active secretory SMase is not necessary for this (8). Holopainen et al (9) also reported the presence of an LDL-associated SMase that may be derived from the apoprotein B of LDL, and which could therefore hydrolyze LDL SM in the circulation. More recently Lee et al (10) reported that cultured endothelial cells and fibroblasts secrete SMase in association with nascent lipoprotein particles, supporting the formation of ceramides in the circulation. The concentration of ceramides is increased in sepsis patients, and the ceramide/SM ratio correlates positively with mortality in the patients (11).
Although the cellular functions of ceramide have been well investigated, its possible effects in the plasma compartment are unknown. Studies from several laboratories, including ours, have shown that ceramide is an activator of secretory and cytosolic phospholipases in vitro (12–16). Because of its tendency to form inverted hexagonal phase structures (HII), it disrupts membrane bilayer (12,17), and this may be the basis for its activation of phospholipase activities. Furthermore, ceramide was reported to specifically stimulate the release of unsaturated fatty acids from phospholipids by sPLA2 IIa (13), suggesting that it facilitates the interaction of lipolytic enzymes with specific phospholipid species. The possible effect of ceramide on the activity and specificity of LCAT, an enzyme that is essentially a modified PLA2, has not been investigated, although SM, its precursor, has been shown to inhibit LCAT activity (18–21). In this study, we addressed the effect of SM and its metabolites, ceramide and ceramide phosphate, on the activity and fatty acid specificity of human LCAT. The results show that SM and ceramide have opposing effects on the enzyme activity, and that ceramide phosphate is a more potent inhibitor of the enzyme than SM. Furthermore, ceramide stimulated the transfer of polyunsaturated fatty acids to cholesterol, at the expense of saturated fatty acids. This function of ceramide may be important in the regulation of LCAT specificity, and could possibly influence the atherogenic properties of the lipoproteins.
Materials and Methods
Materials
Egg PC, human serum albumin, egg ceramide, and unlabeled cholesterol and SMase C (S.aureus, 174 units/mg protein) were obtained from Sigma-Aldrich. Labeled cholesterol (4-[14C], 53mCi/mmol), and labeled PC (1-palimtoyl 2-[1-14C]-linoleoyl PC, 52 mCi/mmol) were purchased from Amersham Biosciences. Dioctadecenyl PC (18:1 diether PC), and synthetic PCs (16:0–16:0, 16:0–18:1, 16:0–18:2, 16:0–20:4) were purchased from Avanti Polar Lipids. Recombinant SMase D from C. pseudotuberculosis was purified from the transfected E.Coli, as described previously (22). This enzyme was stored at −30 °C in 50% glycerol at a protein concentration of 100 μg/ml. The specific activity of this preparation was 4.6 units/ml, using the Amplex Red SMase assay kit from Molecular Probes, Inc (1 unit hydrolyzes 1.0 μmol of SM per min at 37 °C). It has no activity towards PC or other diacyl phosphoglycerides, although it showed some activity against lyso PC. Ceramide phosphate was prepared by treating egg SM liposomes containing 10 mol% of egg phosphatidylethanolamine (PE) with recombinant SMase D, and purified by silica gel TLC in the solvent system of chloroform: methanol: water (65:25:4 v/v). The presence of PE was necessary for the optimal hydrolysis of PC, although PE itself was not hydrolyzed by the enzyme.
LCAT and apo AI preparation
LCAT was purified, as described previously (23), from normal human plasma obtained from the local blood bank. For most experiments the phenyl Sepharose eluate was used instead of the more purified enzyme, because of the instability of the pure enzyme. The major contaminant in the phenyl Sepharose eluate was albumin, which is a normal constituent of the reaction mixture. The amounts of PC, as measured by lipid phosphorus (24) and cholesterol (measured by enzymatic kit from Waco) in the enzyme preparation were negligible in the sample (the detection limits were was 50 ng and 1 μg respectively for lipid phosphorus and cholesterol). Apoprotein AI was purified from normal human HDL, as described previously (25).
Substrate preparation
Proteoliposome substrates were prepared by a modification of the method of Chen and Albers (23). Briefly, 375 nmol of PC, 18.75 nmol of unlabeled cholesterol, 0.3 μCi 14C-cholesterol, and varying amounts of sphingolipids, where indicated, were added into conical bottom screw-cap glass tube and the solvent was evaporated under nitrogen. The lipids were re-dissolved in 120 μl ethanol and dried again under nitrogen. The sample was then dispersed in 12 μl 750 mM Na-cholate in Standard Buffer (0.15 M NaCl, 10 mM Tris-Cl, pH 7.4, 1 mM EDTA and 0.05% sodium azide). To the resulting dispersion, 30,0 μl of Standard Buffer was added and the sample was incubated at 60 ºC for 10 min, then at 37 ºC for 10 min. To the cooled sample 30.0 μg apoA1 in Standard Buffer was added and the final volume was adjusted to 150 μl with Standard Buffer. Samples were vortexed for 1 min, and incubated for 30 min at 37 ºC. Sodium cholate was removed by extensive dialysis against 3x 1 l Standard Buffer (overnight at 4ºC, 7–8 h at RT and finally 2 days at 4 ºC).
Diether PC-containing proteoliposomes were prepared as described above, except that 80% of PC was replaced by dioctadecenyl (di-18:1) ether PC. The amount of cholesterol was decreased accordingly to keep the (diester) PC: cholesterol molar ratio at 20:1. The standard reaction mixture contained 75 nmol PC, 3.75 nmol cholesterol and 300 nmol di-18:1 PC ether in 150 μl). Proteoliposomes for the determination of phospholipase A activity of LCAT in the ether matrix were prepared as described above, excepting that 0.5 μCi 16:0-[1-14C]18:2 PC was used as the label, and cholesterol was omitted.
Assay of the LCAT activity
LCAT activity was measured in a total volume of 200 μl containing 2.5 mg/ml human serum albumin, 10 mM β-mercaptoethanol, 20 μl of proteoliposome preparation (containing 50 nmol of PC and 2.5 nmol of labeled cholesterol unless indicated otherwise) and 10–30 μl (5–15 μg protein) of the enzyme preparation (phenyl Sepharose eluate). The amount of the enzyme was adjusted to give a 15–25% esterification of labeled cholesterol in 30 min. The reaction was stopped by adding 800 μl ethanol, and the lipids were extracted with 2 × 2 ml hexane, containing 25 μg/ml cholesterol and 25 μg/ml cholesteryl oleate as carriers. The lipids were separated on a silica-gel TLC plate using petroleum ether: ethyl acetate (85:15, v/v) as the mobile phase. Cholesterol and cholesterol ester bands were scraped from the plate and their radioactivity was measured in a liquid scintillation counter.
Assay of phospholipase activity
The reaction conditions for phospholipase activity of LCAT were similar to the cholesterol esterification assay, excepting that the substrate contained labeled PC (16:0-[1-14C]-18:2 PC) and did not contain cholesterol, unless otherwise indicated. The reaction was stopped by the addition of 1 ml methanol containing 100 μg cholesteryl oleate and 100 μg free oleic acid, and the samples were extracted by Bligh and Dyer method (26). The lipids were separated on silica-gel TLC plate using hexane: ethyl acetate: acetic acid (80:20:1, v/v) as mobile phase. PC, cholesteryl ester and free fatty acid bands were scraped from the plate and their radioactivity determined in a liquid scintillation counter.
SMase treatments
When the SM in the substrate was hydrolyzed before reaction with LCAT, the proteoliposome substrate was first incubated (for 30–60 min) with the indicated concentration of SMase C or SMase D at pH 7.4 in Standard Buffer in presence of 0.2 mM Mg2+ and 0.05 mM Mn2+, in a final volume of 0.2 ml. The reaction was stopped by the addition of 4 mM EDTA in 20 mM phosphate buffer, pH 7.5, following which, human serum albumin, mercaptoethanol, and LCAT were added as described for the cholesterol esterification assay, and the incubation continued for another 30 min in a final volume of 0.4 ml. The LCAT reaction was stopped by the addition of 1 ml methanol, the lipids were extracted by Bligh and Dyer procedure (26), and processed as described above.
Determination of fatty acid composition
For the determination of the composition of free fatty acids released by LCAT, the reaction mixture was scaled up by using 120 μl of proteoliposome substrate (containing 300 nmol of total PC, but no cholesterol). Composition of the reaction mixture was identical to that used for the LCAT activity measurement, excepting that the reaction volume was increased to 400 μl, the amount of enzyme was increased 10 times and incubation time extended to 60 min. In some experiments, the substrate was completely hydrolyzed with snake venom phospholipase A in order to determine the sn-2 fatty acid composition of the PC substrate used. This was performed in identical reaction mixture as LCAT, but supplemented with 10 mM Ca2+ and 1.2 units of Naja mozambique phospholipase A2 and 12 μl of standard proteoliposome preparation (containing 30 nmol of total PC).
The reactions were stopped by the addition of 1.6 ml ethanol, and the lipids extracted with 2 × 4 ml hexane after adding 2 μg of heptadecanoic acid (17:0) as a carrier and internal standard. The solvent was evaporated under nitrogen, and the lipids separated on a silica-gel plate using solvent system of hexane: diethyl ether: acetic acid (70:30:1, v/v) or hexane: ethyl acetate: acetic acid (80:20:1, v/v). The free fatty acid spot was identified by exposing only the lane containing the oleic acid standard (100 μg) to iodine vapors. Area corresponding to the free fatty acid was scraped from each sample lane and transferred into a screw-cap glass tube. The fatty acids were methylated with an instant methanolic HCl kit (acetyl chloride in methanol, Alltech Associates), according to manufacturer’s protocol. Water (1 ml) was added, and the fatty acid methyl esters were extracted twice with 2 ml hexane. The solvent was evaporated, the sample re-dissolved in 20 μl hexane and 3 μl of it was injected into a Shimadzu GC-17A, equipped with a flame ionization detector. The fatty acid methyl esters were analyzed under the conditions described previously (27). The concentration of individual fatty acids were calculated with the help of the internal standard (17:0).
Determination of fatty acid specificity of LCAT
For the analysis of CE species formed by LCAT, the reaction mixture was scaled up to contain 50 μl of proteoliposome preparation (125 nmol of PC and 6.25 nmol cholesterol), and the lipids were extracted as described for standard LCAT assay, after which 10% of the lipid extract was used for the determination of the total enzyme activity. The remainder was concentrated under nitrogen, and loaded onto a C-18 reverse phase HPLC column. CE species were separated from each other and from free cholesterol using the solvent system of acetonitrile : tetrahydrofuran : water (65:35:1.9, v/v), and the radioactivity of the peaks was determined online with a radioactivity detector, as described previously (28).
Results
Effect of SMase C and SMase D on LCAT activity
Although previous studies from our laboratory and others showed that SM is a physiological inhibitor of LCAT reaction (18–21), the structural requirements for this inhibition were not investigated. As a first step in understanding the molecular requirements for LCAT inhibition, we treated the SM-containing proteoliposome substrate with either SMase C, which hydrolyzes SM to ceramide, or with SMase D, which hydrolyzes it to ceramide phosphate (see Scheme 1), and then determined the effect on LCAT activity. As shown in Fig. 1, the presence of SM at 50 mol% of PC resulted in about 65% inhibition of the activity, and this inhibition was abolished by pre-treatment of the substrate with SMase C, in agreement with our previous studies (18). Compared to the untreated control, SMase C treatment resulted in a 3-fold stimulation of the enzyme activity. In contrast, when the substrate was treated with SMase D before incubation with LCAT, there was a further inhibition of LCAT activity, compared to the untreated sample. About 80% of SM was hydrolyzed under these conditions by both SMases. When the substrate was first treated with SMase C, and then with SMase D, there was no inhibition of LCAT activity, showing that SMase D has no direct effect on LCAT activity. Since most of the SM was converted to ceramide before the addition of SMase D, these results also show that the formation of ceramide phosphate was necessary for the increased inhibition of LCAT by SMase D. When the substrate was first treated with SMase D, and then with SMase C, the LCAT reaction was inhibited, showing that the depletion of SM is not enough, but the formation of ceramide was necessary, for the activation to occur. Treatment of SM-free liposomes with SMase D did not affect the LCAT activity (results not shown), supporting the conclusion that the formation of ceramide phosphate was necessary for the inhibition.
Scheme 1.
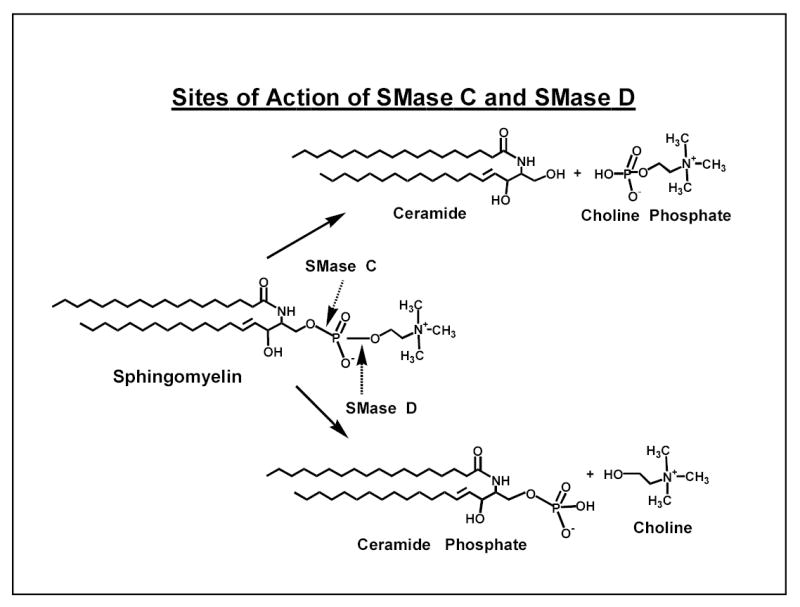
Figure 1. Effect of pre-treatment of SM-containing substrate with SMase C or D on LCAT activity.
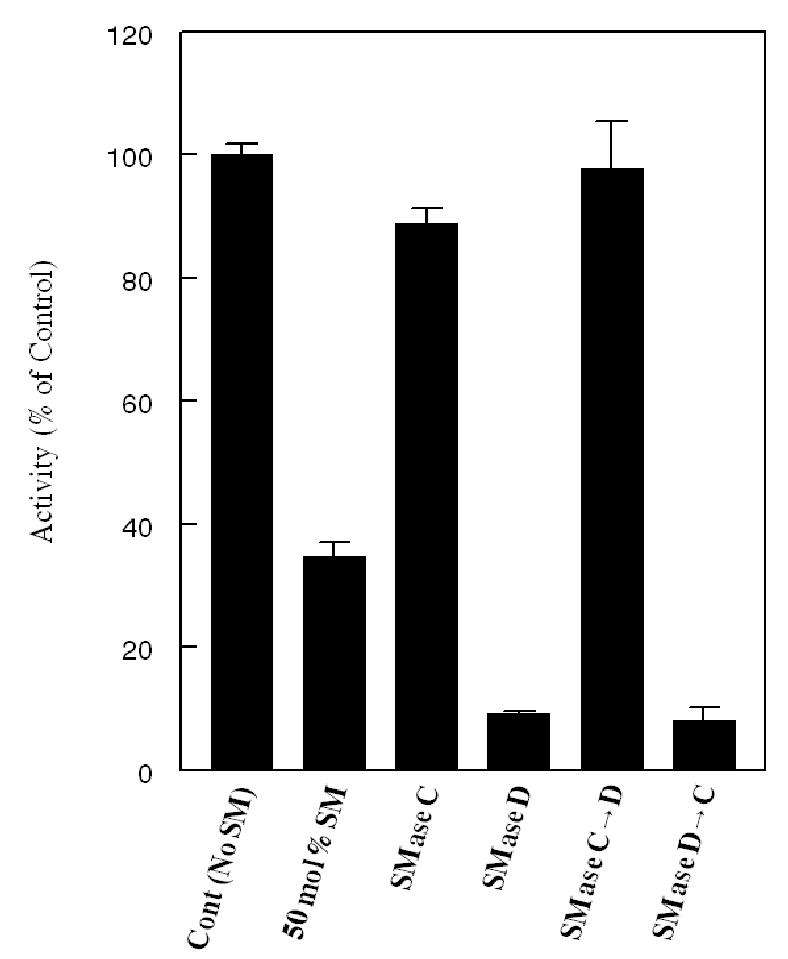
Egg PC-[14C] cholesterol proteoliposomes containing 50 mol% SM (with respect to PC) were first treated for 30 min with either SMase C (100 mU) or SMase D (100 mU), or a sequential combination of the two, in the presence of 50 μM Mn2+ and 200 μM Mg2+, as described in Methods. The SMase reaction was stopped by the addition of 4 mM EDTA, and the substrate was then incubated with human LCAT (phenyl Sepharose eluate) for an additional 30 min. The LCAT reaction was stopped by the addition of ethanol containing unlabeled FC and CE, and the percent of labeled FC esterified was determined as described in Methods. All activities are expressed as percent of activity obtained with SM-free substrate. The values shown are mean ± SEM of 4–6 experiments.
Correlation of LCAT activity with degradation of SM
To further establish that the differential effects of the two SMases is due to the different products formed rather than due to different levels of SM hydrolysis, we compared the amount of SM remaining after treatment with increasing concentrations of SMase C or SMase D, with the subsequent effect on LCAT activity. For studying the SMase C effect, we used a substrate that contained 50 mol% SM (relative to PC), whereas for the SMase D effect, we used a substrate that contained 35 mol% SM. We used different initial concentrations of SM because we expected the LCAT activity to be stimulated by SMase C, but inhibited further by SMase D. It is therefore necessary to start with lower initial inhibition of LCAT activity in the case of SMase D, in order to show further inhibition by this enzyme. As shown in Fig. 2, LCAT reaction was activated in proportion to the SM hydrolyzed by SMase C, whereas it was inhibited in parallel with the SM hydrolysis by SMase D. It is noteworthy that the full enzyme activity (i.e., the activity observed in the absence of SM) was restored after only a partial hydrolysis of SM by SMase C, suggesting that ceramide may either independently stimulate LCAT reaction, or neutralize the effect of the remaining SM. The data in Fig. 2 show that the full activity was restored after treatment with 120 mU of SMase C, although the SM remaining in the reaction mixture was about 32 mol% of PC. This amount of SM by itself (in the absence of SMase C reaction) normally inhibits LCAT activity by about 40% (see the control value for SMase D studies at the bottom). Treatment with higher amounts of SMase C did not further stimulate LCAT activity, and in fact inhibited the reaction by about 15%, although the SM content was reduced to less than 10 mol% of PC (result not shown).
Figure 2. Correlation of LCAT activity with the extent of SM hydrolysis by SMase C or SMase D.
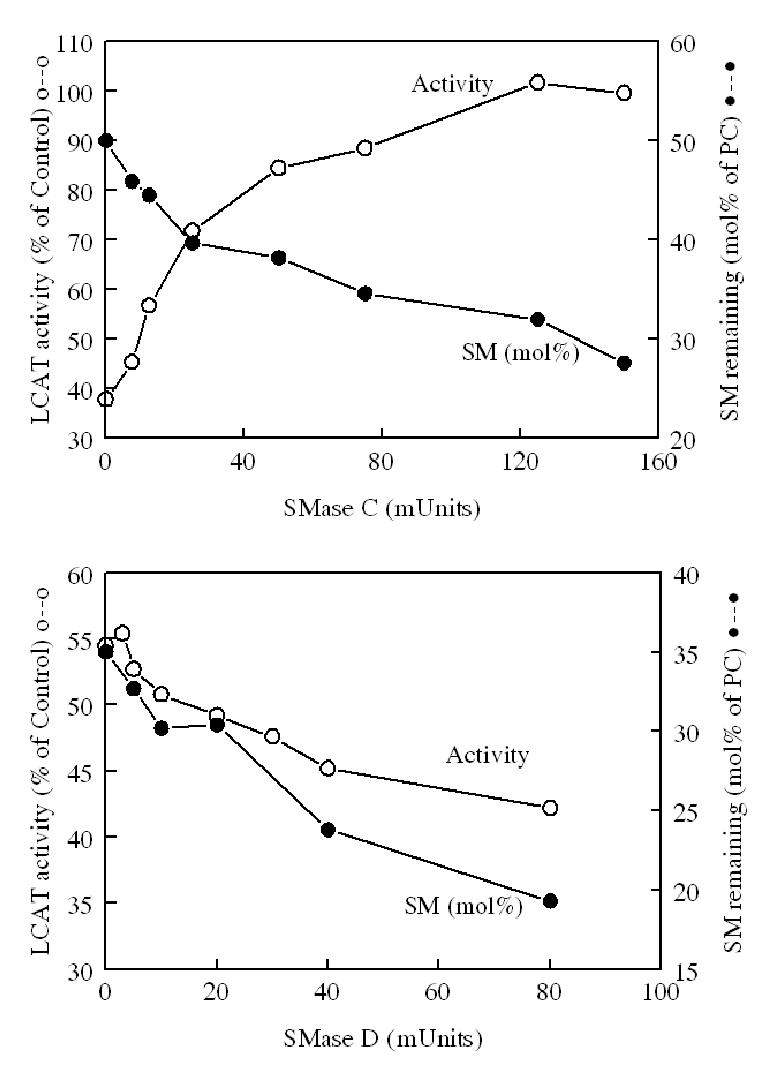
Top panel (SMase C): Egg PC-[14C] FC proteoliposomes containing 50 mol% SM (with respect to PC) were treated with varying amounts of SMase C for 30 min in the presence of 50 μM Mn2+ and 200μM Mg2+. The SMase reaction was stopped by the addition of 4 mM EDTA, and the sample then incubated with LCAT for 30 min. LCAT activity was determined as described in Methods. The extent of SM hydrolysis was determined by estimating the amount of SM remaining in separate aliquots of reaction mixture treated identically with SMase. For this purpose, the lipids were extracted by Bligh and Dyer procedure (26), the lipid extract was separated on silica gel TLC plates with the solvent system of chloroform: methanol: water (65: 25: 4 v/v), and the spot corresponding to authentic SM was scraped, and the lipid phosphorus determined by the modified Bartlett procedure (24). The LCAT activity is expressed as percent of the activity obtained with SM-free substrate. The values shown are averages of duplicate samples.
Bottom panel (SMase D): Egg PC: [14C]- cholesterol liposomes containing 35 mol% SM (with respect to PC) were treated with varying concentrations of recombinant SMase D for 30 min, and the LCAT activity and SM levels were determined as described above for SMase C. All values shown are means of two separate experiments.
Effect of ceramide and ceramide phosphate
The results described above strongly suggest that the enzymatic generation of ceramide activates the LCAT reaction, whereas the generation of ceramide phosphate inhibits it. To investigate the effects of ceramide and ceramide phosphate directly, we incorporated varying amounts of these lipids into the proteoliposomes in the absence of SM, and determined the effect on LCAT activity. As shown in Fig. 3, low concentrations of ceramide (< 0.2 mol% of PC) stimulated the LCAT activity by about 15%, but higher concentrations either had no effect or inhibited the enzyme. Ceramide phosphate, on the other hand, had no stimulatory effect at any concentration, but consistently inhibited the reaction above 1 mol% of PC. At a ceramide phosphate concentration of 25 mol% of PC, the enzyme activity was inhibited by 80%. In comparison, SM at 25 mol% inhibited the reaction by only about 25–35% (see Figs. 4 and 5). Thus ceramide phosphate is a stronger inhibitor of LCAT than SM at equivalent concentration, and this accounts for the inhibitory effect of SMase D on SM-containing substrate.
Figure 3. Effect of ceramide or ceramide phosphate in SM-free proteoliposomes.
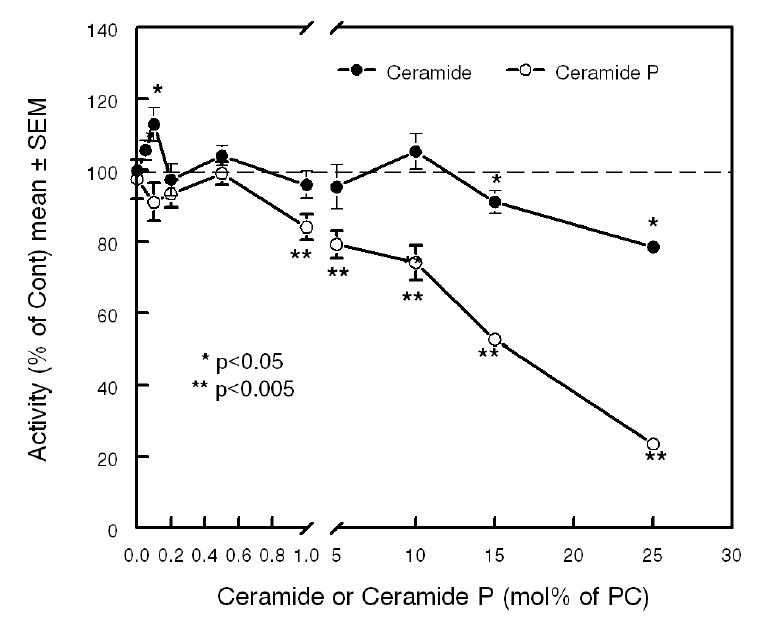
Substrates containing the indicated mol% of ceramide (closed circles) or ceramide phosphate (open circles) were prepared as described in Methods. The esterification of labeled cholesterol by LCAT was measured as described in the text. Values shown are mean ± SEM of at least 3 experiments.
* p< 0.05, ** p< 0.005 comparison with sphingolipid-free substrate.
Figure 4. Effect of incorporation of ceramide into SM-containing substrate.
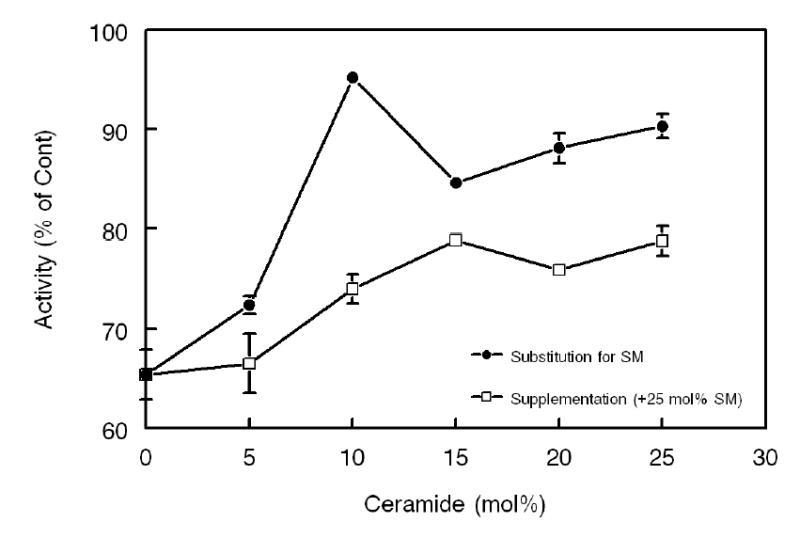
Open squares indicate the substrates in which the indicated mol% of ceramide was incorporated in addition to the 25 mol% SM (supplementation). Closed circles indicate substrates in which the indicated mol% of ceramide replaced equimolar amounts of SM, keeping the total sphingolipid concentration at 25mol% of PC (substitution). All enzyme activities are expressed as percent of activity measured in the absence of any sphingolipid (mean ± SEM of 3 experiments).
Figure 5. Comparative effects of sphingolipids on the two steps of LCAT reaction.
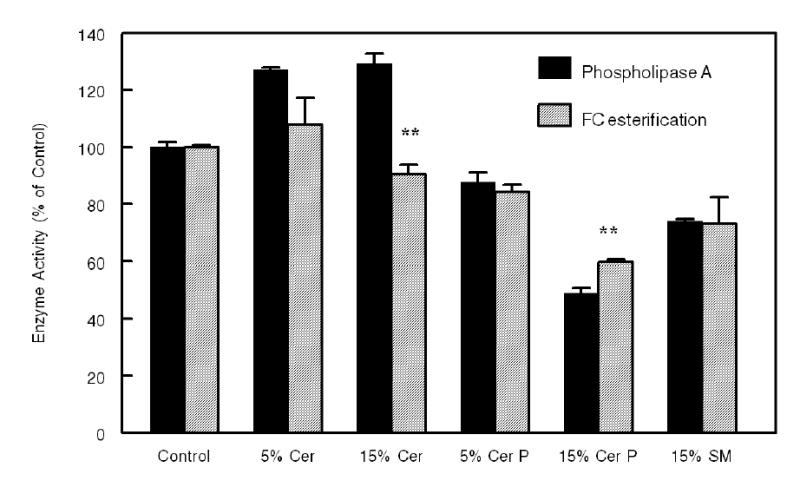
Proteoliposome substrates containing 16:0-[1-14C]-18:2 PC and unlabeled cholesterol (at PC: cholesterol molar ratio of 20:1), and the indicated mol% of sphingolipid, were prepared as described in the text. After incubation with purified LCAT for 30 min, the lipids were extracted by ethanol/hexane, and the radioactivity in free fatty acids and CE was determined as described in Methods. All activities are expressed as percent of the activity obtained in the absence of added sphingolipid. Values shown are mean ± SEM of at least 3 experiments. Where indicated (** p< 0.005), the effect on phospholipase A activity was significantly different from the effect on FC esterification activity.
Effect of ceramide in presence of SM
Results in Fig. 2 show that partial hydrolysis of SM restored the LCAT activity to that of SM-free substrate, whereas the results in Fig. 3 show that ceramide is not a strong activator of LCAT in the absence of SM. This raises the possibility that ceramide counteracts the effect of SM, rather than directly activating the enzyme. To test this possibility, we incorporated increasing amounts of ceramide into the proteoliposome substrate containing 25 mol% SM, either as a supplement, or as a substitute for SM. The latter condition simulates the results of SMase C reaction, and keeps the total sphingolipid concentration at 25 mol% of PC. As shown in Fig. 4, the enzyme activity was inhibited by about 35% in the presence of 25 mol% SM alone. When ceramide was added as a supplement (i.e., without decreasing the SM concentration), there was a partial restoration of the activity (up to 80% of the SM-free control) at 15 mol% ceramide (+25 mol% SM, bringing the total sphingolipid concentration to 40 mol%). When ceramide replaced SM, however, almost complete restoration of the activity was observed with only partial replacement (10 mol% ceramide + 15 mol% SM). Replacing further amount of SM with ceramide showed no further stimulation. It may be noted that 15 mol% SM, in the absence of ceramide, inhibits the enzyme activity by 20–30% (see Fig 5). Since this inhibition is abolished by the presence of 10 mol% ceramide, these results show that ceramide and SM have opposing effects on LCAT activity.
Effect of sphingolipids on the phospholipase A activity of LCAT
LCAT reaction takes place in two distinct steps, namely the formation of an acyl-enzyme intermediate with the acyl group of PC and the active site serine, followed by the transfer of the acyl group to cholesterol. In the absence of sufficient amount of cholesterol or other acyl acceptor, the acyl group is released as free fatty acid (phospholipase A reaction) (29). The first step of the reaction can, therefore, be studied as the phospholipase A activity by measuring the release of labeled free fatty acid from labeled PC. Previous studies showed that ceramide is an activator of various phospholipase reactions due to its bilayer-disrupting properties (12,13,15,16). Recent studies also show that ceramide influences the distribution of cholesterol in the membrane bilayer, by specifically displacing it from the raft domains (30,31). It is, therefore, possible that ceramide has independent effects on the two steps of the LCAT reaction. To test this possibility, we prepared proteoliposome substrates with 16:0-[1-14C -18:2]-PC in the presence of 5 mol% cholesterol. Under these conditions, both phospholipase A and cholesterol esterification can be measured (25). The effect of incorporation of ceramide, ceramide phosphate or SM into this substrate on the two reactions of LCAT was investigated. As shown in Fig. 5, ceramide at 5 mol% and 15 mol% activated the phospholipase A activity by 25–30%, but had much lower effect on cholesterol esterification. In fact, the higher concentration of ceramide slightly inhibited cholesterol esterification. Ceramide phosphate inhibited both reactions to the same extent at low concentration, but inhibited phospholipase activity more at higher concentrations. SM, at 15 mol% inhibited both reactions to the same extent. It should be pointed out since cholesterol esterification is the sum of both reactions, the inhibition of the second step by ceramide may be greater than apparent here, since the first step is actually stimulated by it.
Effect of ceramide on the fatty acid specificity of LCAT
Studies by Koumanov et al (13) showed that ceramide influences the fatty acid specificity of group IIa sPLA2, selectively stimulating the release of polyunsaturated fatty acids from phosphatidyl ethanolamine/phosphatidyl serine (PE/PS) mixtures. To investigate whether ceramide also influences the CE species formed by LCAT, we prepared proteoliposomes with defined PC composition and determined the CE species formed after reaction with LCAT. The substrate contained an equimolar mixture of four common PC species of human plasma (16:0–18:2, 16:0–18:1, 16.0–20:4, and 16:0-16:0), [14C]- cholesterol, apo AI, and 0, 5, or 15 mol% ceramide (relative to PC). After incubation with LCAT, the labeled CE species formed were separated by HPLC, and their radioactivity determined as described in Methods. As shown in Fig. 6, the presence of ceramide significantly stimulated the synthesis of 18:2 CE and 20:4 CE, while it had no influence on 18:1 CE. The synthesis of 16:0 CE was significantly inhibited by the presence of ceramide. These results are similar to the effect of ceramide on sPLA2 IIa hydrolysis of PE/PS mixture, where the release of 20:4 and 18:2 was stimulated more than the release of 18:1 (13). It may be pointed out that in the present study, the formation of 16:0 CE was greater than expected from the percent of 16:0-16:0 PC in the mixture (25%), although LCAT is not known to be specific for this PC species (32), (33). This disproportionate synthesis of 16:0 CE is due to the presence of sn-2-16:0 PC isomers as impurities in the synthetic PCs used. Thus, 16:0–18:2 PC, 16:0–18:1 PC and 16:0–20:4 PC, each had 7–15% of their sn-2 position occupied by 16:0, as determined by the snake venom PLA2 treatment. Consequently, the total 16:0 present at the sn-2 position of the PC mixture was about 33%, instead of 25%. In addition, we have previously shown that human LCAT transfers some 16:0 from the sn-1 position of 16:0–20:4 PC (25,34). While these factors resulted in the formation of higher than expected percent of 16:0 CE, the presence of ceramide significantly decreased 16:0 CE synthesis, suggesting a specific inhibitory effect of ceramide on the synthesis of saturated CE. However, since the total activity of the enzyme was stimulated by about 30% in the presence of ceramide in these experiments, the net effect is the stimulation of synthesis of 20:4 CE and 18:2 CE, rather than an inhibition of 16:0 CE synthesis.
Figure 6. Effect of ceramide on the fatty acid specificity of LCAT reaction.
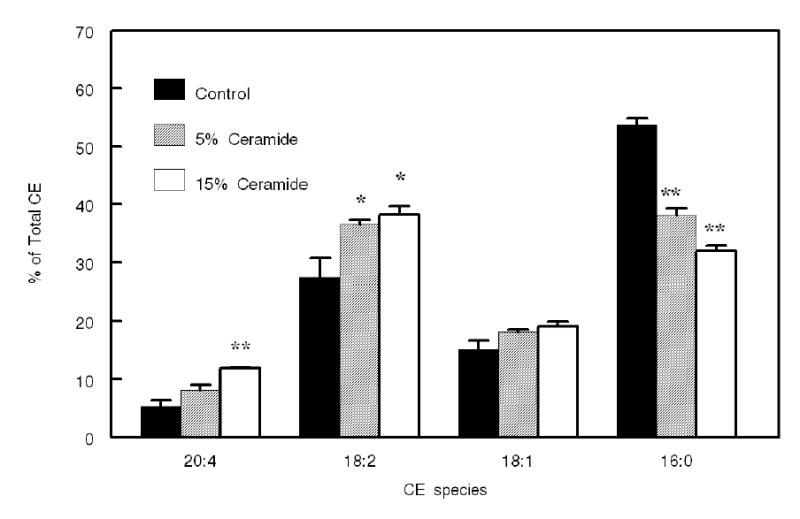
Proteoliposomes containing an equimolar mixture of 16:0-16:0 PC, 16:0–18:1 PC, 16:0–18:2 PC, and 16:0–20:4 PC, labeled cholesterol (at total PC: FC ratio of 20:1), and 0, 5, or 15 mol% ceramide (with respect to total PC), were prepared as described in Methods. The reaction mixture was scaled up to contain 125 nmol of PC and 6.25 nmol of labeled cholesterol per reaction. After lipid extraction, 10% of the lipid extract was taken for the determination of total enzyme activity by TLC, and the rest was used for the separation of CE species by reverse phase HPLC as described in Methods. The activity of the enzyme (pmol CE formed/30 min) in this experiment was 412 ± 32 for control, 553 ± 47 for 5 mol% ceramide, and 606 ± 56 for 15 mol% ceramide. Values shown are mean ± SEM of 3 experiments. Statistical significance of difference between control and ceramide-containing substrates was determined by paired t test.
* p<0.05, ** p< 0.005.
Effect of ceramide on fatty acid specificity of the phospholipase reaction of LCAT
In order to determine whether ceramide influenced the fatty acid specificity at the formation of the acyl-enzyme intermediate or at the cholesterol esterification step, the effect of incorporation of ceramide on the composition of fatty acids released from a defined mixture of PC species (in the absence of cholesterol) was tested. Varying amounts of egg ceramide were incorporated into proteoliposome substrate that contained an equimolar mixture of 16:0–16:0 PC, 16:0–18:1 PC, 16:0–18:2 PC and 16:0–20:4 PC, and after reaction with LCAT, the composition of the fatty acids released was determined by gas liquid chromatography as described in Methods. The percent of individual fatty acids released in the presence of ceramide was calculated relative to its percentage in the absence of ceramide. As shown in Fig 7, the release of both 20:4 and 18:2 was stimulated by the presence of ceramide. Since the percent stimulation is comparable to that observed for cholesterol esterification (see Fig. 6), the specificity effect of ceramide appears to be exerted at the first (acyl-enzyme intermediate formation) step of the LCAT reaction. The release of 16:0 was inhibited by the presence of ceramide, but this effect was less than that observed for cholesterol esterification.
Figure 7. Effect of ceramide on the fatty acid specificity of the phospholipase A reaction of LCAT.
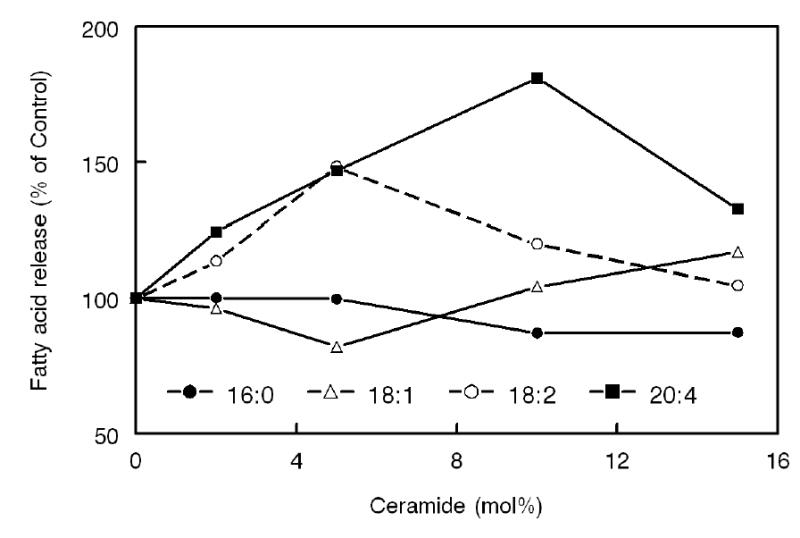
Varying amounts of ceramide were incorporated into proteoliposomes containing an equimolar mixture of 16:0-16:0 PC, 16:0–18:1 PC, 16:0–18:2 PC, and 16:0–20:4 PC, in the absence of cholesterol. After reaction with LCAT, the released free fatty acids were analyzed by gas liquid chromatography, using 17:0 free fatty acid as internal standard. The concentration of individual fatty acids released was calculated as percent of the amount released in the absence of ceramide. Values shown are averages of two experiments.
Influence of matrix on the ceramide effect
The individual PCs used in the above experiment differ significantly in their physical properties and, therefore, it is possible that the effect of ceramide on the fatty acid specificity of LCAT is due to its macromolecular effects on the proteoliposome rather than on the interaction of the individual PC molecules with the active site. In order to minimize the physical differences in the PCs, we incorporated the 4 PCs at equimolar concentration into di-18:1 ether PC matrix, which provides an inert milieu for the substrate PCs (35). The diether PC made up 80% of the total phospholipid, while the four diester PCs constituted 5% each of the total. When ceramide was incorporated into this substrate at either 5 mol% or 15 mol% of the total PC, and the CE species formed by LCAT were analyzed, the differential effect of ceramide on the various CE species disappeared (Fig. 8). These results indicate that the major effect of ceramide is on the macromolecular structure of the substrate, and that when this effect is minimized by the use of PC ether matrix, the ceramide effect on fatty acid specificity is also eliminated.
Figure 8. Effect of ceramide on LCAT fatty acid specificity in the presence of diether PC matrix.
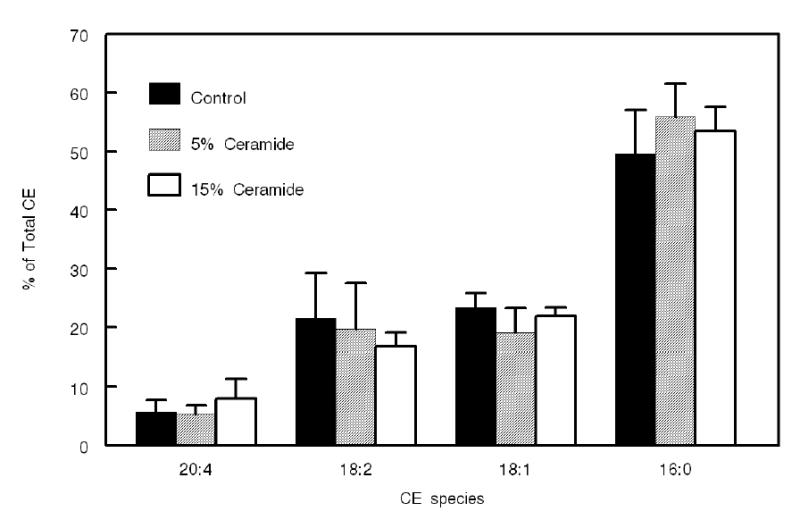
The substrate preparation was similar to that shown in Fig. 6, excepting that 80% of the PC was replaced by dioleyl ether PC, which provides an inert matrix for the substrate. The ratio of diacyl PC : labeled FC was maintained at 20:1 and the mol% of ceramide was calculated with respect to diacyl PC only. The analysis of CE species composition by HPLC was as described in the text. Values shown are mean ± SEM of 3 separate experiments. The total activity of the enzyme (in pmol of CE formed/30 min) was 156 ± 4 (control), 198 ± 7 (5 mol% ceramide), and 142 ± 5 (15 mol% ceramide). None of the CE species showed significant difference (by Student’s t test) between control and ceramide-containing substrates.
Discussion
LCAT reaction is responsible for the synthesis of most of the cholesteryl esters present in human plasma, and is a critical component of the reverse cholesterol transport pathway (29,36). The plasma levels of HDL are positively correlated with this enzyme activity, and therefore the regulation of its activity is of great clinical interest. Furthermore, since different CE species appear to have different atherogenic properties (37,38), the regulation of its fatty acid specificity is also of importance. The physiological factors involved in the regulation of LCAT activity and fatty acid specificity are poorly understood. Previous studies from our laboratory (18), as well as others (19–21) showed that SM, a key phospholipid constituent of all lipoproteins, is a physiological inhibitor of the LCAT reaction. Depletion of SM by bacterial SMase C stimulates the reaction significantly in both native lipoproteins and synthetic substrates (18), supporting the inhibitory role of SM in the lipoproteins. A recent report by Lee et al (10) showed that HDL from Niemann-Pick Disease patients, which contains high concentration of SM, also exhibits impaired cholesterol esterification by LCAT, and that this is mostly attributable to the lack of secretory SMase in these patients. However, studies with various phospholipases showed that ceramide, the product of SMase C reaction, is an independent activator of the phospholipases (12–16). Since LCAT is essentially a specialized phospholipase A that utilizes cholesterol as acyl acceptor in place of water, this raised the possibility that the stimulation of LCAT reaction by SMase C treatment of lipoproteins is due to the formation of an activator (ceramide), rather than depletion of the inhibitor (SM). The availability of recombinant SMase D, which degrades SM without the generation of ceramide provides a new tool to address this question (22) (see Scheme 1). Our previous studies showed, for instance, differential effects of SMase D and SMase C in cultured cells on the metabolic fate of the plasma membrane cholesterol, suggesting an independent effect of ceramide (22). The results presented here show that the degradation of SM by the two SMases yields diametrically opposite effects on the LCAT reaction. While degradation by SMase C activated the reaction by up to 3-fold and completely reversed the inhibitory effect of SM, degradation by SMase D inhibited it further, indicating that the depletion of SM alone does not account for the effects of SMase C. The physiological significance of these findings is not clear because while the presence of ceramide in the lipoproteins has been established (6–8), the presence of ceramide phosphate has not been demonstrated. Nevertheless, these findings offer a clue to the mechanism of the action of sphingolipids on LCAT. First, they show that the presence of choline is not necessary for the SM inhibition of LCAT. Secondly, the presence of phosphate moiety is critical because while ceramide is an activator of the reaction, ceramide phosphate is a more potent inhibitor than SM. Since ceramide phosphate is a phospholipid, it is likely to be integrated into the membrane bilayer, unlike ceramide which is known to disrupt the bilayer structure (12,17). These results are in sharp contrast to the findings of Pettus et al (39), who reported that ceramide phosphate activates cytosolic PLA2 by direct interaction with the CaLB/C2 domain of the enzyme, as well as by translocation of the enzyme to Golgi apparatus. This interaction of ceramide phosphate with PLA2, however, requires Ca2+. Since the LCAT reaction is carried out in the absence of Ca2+, such an interaction with LCAT is unlikely. Moreover, since the amount of ceramide phosphate required to inhibit LCAT is rather high (> 1mol% of PC), it is more likely to be acting through its effects on the substrate.
An interesting finding in our studies is that while ceramide by itself is only a weak activator of LCAT, it effectively neutralizes the inhibitory effect of SM. Since both ceramide and SM are present in the same lipoprotein particle (7,8), this observation could have physiological significance in the regulation of LCAT activity and substrate specificity. The inhibitory effect of SM on LCAT is due to its competition with PC substrate (18), as well as due to its physical effects on the lipoprotein substrate (19). How exactly ceramide counteracts the effect of SM is not yet clear, but several possibilities could be considered. First, it is known that SM helps retain cholesterol in the ordered domains of the membrane (40),(41), while ceramide specifically displaces cholesterol from the membrane rafts (30,31). It is therefore possible that ceramide offsets the inhibitory effect of SM by ‘liberating’ the SM-sequestered cholesterol to accept the acyl group in the LCAT reaction. Our results, however, show that the major stimulatory effect of ceramide is on the first step of the reaction (acyl-enzyme intermediate formation) rather than on the acyl transfer step. Furthermore, the inhibitory effect of SM is also predominantly on the first step, because the phospholipase A reaction of the enzyme is as sensitive as cholesterol esterification reaction (Fig. 5) (18), even though SM is known to specifically interact with cholesterol molecule (41,42). Thus it appears unlikely that the ceramide effect is due to the displacement of cholesterol from the ordered domains of the membrane. A second possibility is that ceramide counteracts the effects of SM by disordering the membrane structure and thereby allowing an increased penetration of the enzyme into the bilayer. Since the membrane is less ordered to begin with in the absence of SM, this effect of ceramide would not be noticeable in the absence of SM. Thirdly, the long chain ceramides are known to induce lateral phase separation of the PC bilayer into gel and liquid crystalline domains (12). Such phase separation could lead to the formation of long-lived boundary regions in which the individual PC molecules are less tightly bound (43) and therefore can dissociate from the bulk phase and interact with the enzyme’s active site. Studies by Holopainen et al (44) also show that while SM inhibits the lateral diffusion rates of lipids in the bilayer, the presence of even a small percent of ceramide offsets this effect. It is therefore possible that the neutralizing effect of low concentration of ceramide on the SM inhibition of LCAT is due to its ability to counteract the inhibitory effect of SM on the lateral diffusion rates. Higher concentrations of ceramide, on the other hand, increase the molecular packing of the PC molecules, in addition to increasing the lateral diffusion rates (44). Since the higher packing density of PC is inhibitory to LCAT reaction (19), this could explain the inhibitory effect of higher ceramide levels both in the presence and absence of SM. Ceramide is also believed to activate some phospholipases by direct interaction with the enzyme (45), but this is unlikely in the case of LCAT because the addition of ceramide separately to the reaction mixture did not stimulate the reaction (results not shown). Furthermore, as shown in Fig. 3, incorporation of ceramide into the substrate (in the absence of SM) activated the reaction by less than 20%, in contrast to the 2–3 fold activation of sPLA2 IIa (13). It also appears unlikely that ceramide acts on apo AI (the protein activator of LCAT), because our earlier studies (18) showed that the inhibition by SM is not due to its effect on apo AI. On the basis of these considerations, we propose that the stimulation of LCAT activity by SMase C treatment is due to membrane reorganization by ceramide rather than due to a simple depletion of SM.
Our studies also show that ceramide stimulates the synthesis of polyunsaturated CE, and inhibits the synthesis of saturated CE. These effects are similar to those reported for sPLA2 activity with a mixture of PE and PS as substrate (13). However unlike the 15-fold increase in the release of polyunsaturated fatty acids reported for sPLA2, the synthesis of 20:4 CE by LCAT was stimulated by only about 2-fold by ceramide. Although the presence of cholesterol has been shown to dampen the effect of ceramide on the PLA2 specificity (13), it is unlikely to be a factor in case of LCAT, because even in the absence of cholesterol, the release of 20:4 as free fatty acid (phospholipase A activity) was only stimulated by about 2-fold (Fig. 7). Interestingly, the specificity effect is abolished when the PC substrates were incorporated into an inert PC diether matrix, which minimizes the differences in the physical properties of the various PC substrates. This strongly suggests that ceramide affects the distribution of PC molecular species on the surface of the substrate particle, possibly through the microdomain formation. On the basis of small angle x-ray refraction studies, Koumanov et al (13) suggested that the long chain polyunsaturated PCs concentrate in the intermediate structures between the hexagonal and lamellar phases created by the ceramide-induced phase separation, and that these structures are more susceptible to the enzymatic attack. It is conceivable that the enrichment of lipoproteins with ceramide, either through SMase C action or through direct secretion from the tissues (7,8,10) could facilitate interaction of more polyunsaturated PCs with LCAT. Our previous studies also showed that the action of LCAT on 16:0–20:4 PC results in the formation of 20:4 lyso PC, due to an alteration in its positional specificity (25). Therefore an increase in ceramide during the inflammatory reactions could theoretically increase the formation of 20:4 lyso PC, which is known to be a preferred source of the pro-inflammatory 20:4 for certain tissues (46).
Acknowledgments
We wish to thank Dr. Dev K. Singh for the preparation of SMase D, and Dr. Robert Bittman (Queens College of CUNY) for helpful comments.
Footnotes
Supported by a grant from National Institutes of Health, HL 68585.
References
- 1.Myher JJ, Kuksis A, Pind S. Molecular species of glycerophospholipids and sphingomyelins of human plasma: Comparison to red blood cells. Lipids. 1989;24:408–418. doi: 10.1007/BF02535148. [DOI] [PubMed] [Google Scholar]
- 2.Subbaiah PV, Rodby RA. Abnormal acyltransferase activities and accelerated cholesteryl ester transfer in patients with nephrotic syndrome. Metabolism. 1994;43:1126–1133. doi: 10.1016/0026-0495(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 4.Park, T. S., Panek, R. L., Mueller, S. B., Hanselman, J. C., Rosebury, W. S., Robertson, A. W., Kindt, E. K., Homan, R., Karathanasis, S. K., and Rekhter, M. D. (2004) Inhibition of Sphingomyelin Synthesis Reduces Atherogenesis in Apolipoprotein E-Knockout Mice, Circulation [DOI] [PubMed]
- 5.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of Myriocin on Plasma Sphingolipid Metabolism and Atherosclerosis in apoE-deficient Mice. J BiolChem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 6.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18:1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 8.Lightle S, Tosheva R, Lee A, Queen-Baker J, Boyanovsky B, Shedlofsky S, Nikolova-Karakashian M. Elevation of ceramide in serum lipoproteins during acute phase response in humans and mice: role of serine-palmitoyl transferase. Arch Biochem Biophys. 2003;419:120–128. doi: 10.1016/j.abb.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Holopainen JM, Medina OP, Metso AJ, Kinnunen PKJ. Sphingomyelinase Activity Associated with Human Plasma Low Density Lipoprotein. Possible functional implications. J Biol Chem. 2000;275:16484–16489. doi: 10.1074/jbc.275.22.16484. [DOI] [PubMed] [Google Scholar]
- 10.Lee CY, Lesimple A, Denis M, Vincent J, Larsen A, Mamer O, Krimbou L, Genest J, Marcil M. Increased sphingomyelin content impairs HDL biogenesis and maturation in human Niemann-Pick disease type B. J Lipid Res. 2006;47:622–632. doi: 10.1194/jlr.M500487-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Huang HW, Goldberg EM, Zidovetzki R. Ceramides perturb the structure of phosphatidylcholine bilayers and modulate the activity of phospholipase A2. European Biophysics Journal. 1998;27:361–366. doi: 10.1007/s002490050143. [DOI] [PubMed] [Google Scholar]
- 13.Koumanov KS, Momchilova AB, Quinn PJ, Wolf C. Ceramides increase the activity of the secretory phospholipase A2 and alter its fatty acid specificity. Biochem J. 2002;363:45–51. doi: 10.1042/0264-6021:3630045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klapisz E, Masliah J, Bereziat G, Wolf C, Koumanov KS. Sphingolipids and cholesterol modulate membrane susceptibility to cytosolic phospholipase A(2) J Lipid Res. 2000;41:1680–1688. [PubMed] [Google Scholar]
- 15.Hashizume T, Kitatani K, Kageura T, Hayama M, Akiba S, Sato T. Ceramide enhances susceptibility of membrane phospholipids to phospholipase A(2) through modification of lipid organization in platelet membranes. Biol Pharm Bull. 1999;22:1275–1278. doi: 10.1248/bpb.22.1275. [DOI] [PubMed] [Google Scholar]
- 16.Gesquiere L, Cho W, Subbaiah PV. Role of group IIa and group V secretory phospholipases A2 in the metabolism of lipoproteins. Substrate specificities of the enzymes and the regulation of their activities by sphingomyelin. Biochemistry. 2002;41:4911–4920. doi: 10.1021/bi015757x. [DOI] [PubMed] [Google Scholar]
- 17.Veiga MP, Arrondo JL, Goni FM, Alonso A. Ceramides in Phospholipid Membranes: Effects on Bilayer Stability and Transition to Nonlamellar Phases. Biophys J. 1999;76:342–350. doi: 10.1016/S0006-3495(99)77201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbaiah PV, Liu M. Role of sphingomyelin in the regulation of cholesterol esterification in the plasma lipoproteins. Inhibition of lecithin- cholesterol acyltransferase. J Biol Chem. 1993;268:20156–20163. [PubMed] [Google Scholar]
- 19.Bolin DJ, Jonas A. Sphingomyelin inhibits the lecithin-cholesterol acyltransferase reaction with reconstituted high density lipoproteins by decreasing enzyme binding. J Biol Chem. 1996;271:19152–19158. doi: 10.1074/jbc.271.32.19152. [DOI] [PubMed] [Google Scholar]
- 20.Rye KA, Hime NJ, Barter PJ. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J Biol Chem. 1996;271:4243–4250. doi: 10.1074/jbc.271.8.4243. [DOI] [PubMed] [Google Scholar]
- 21.Sparks DL, Frank PG, Neville TAM. Effect of the surface lipid composition of reconstituted LpA-I on apolipoprotein A-I structure and lecithin: cholesterol acyltransferase activity. Biochim Biophys Acta. 1998;1390:160–172. doi: 10.1016/s0005-2760(97)00172-0. [DOI] [PubMed] [Google Scholar]
- 22.Subbaiah PV, Billington SJ, Jost BH, Songer JG, Lange Y. Sphingomyelinase D, a novel probe for cellular sphingomyelin: effects on cholesterol homeostasis in human skin fibroblasts. J Lipid Res. 2003;44:1574–1580. doi: 10.1194/jlr.M300103-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Albers JJ. Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. J Lipid Res. 1982;23:680–691. [PubMed] [Google Scholar]
- 24.Marinetti GV. Chromatographic separation, identification, and analysis of phosphatides. J Lipid Res. 1962;3:1–20. [Google Scholar]
- 25.Subbaiah PV, Liu M, Bolan PJ, Paltauf F. Altered positional specificity of human plasma lecithin-cholesterol acyltransferase in the presence of sn-2 arachidonoyl phosphatidyl cholines. Mechanism of formation of saturated cholesteryl esters. Biochim Biophys Acta. 1992;1128:83–92. doi: 10.1016/0005-2760(92)90261-s. [DOI] [PubMed] [Google Scholar]
- 26.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.Subbaiah PV, Sowa JM, Davidson MH. Evidence for altered positional specificity of LCAT in vivo: studies with docosahexaenoic acid feeding in humans. J Lipid Res. 2004;45:2245–2251. doi: 10.1194/jlr.M400197-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Subramanian VS, Subbaiah PV. Modulation of the positional specificity of lecithin-cholesterol acyltransferase by the acyl group composition of its phosphatidylcholine substrate: Role of the sn-1 acyl group. Biochemistry. 1998;39:13626–13633. doi: 10.1021/bi980351e. [DOI] [PubMed] [Google Scholar]
- 29.Jonas A. Regulation of lecithin cholesterol acyltransferase activity. Prog Lipid Res. 1998;37:209–234. doi: 10.1016/s0163-7827(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 30.Megha. London E. Ceramide Selectively Displaces Cholesterol from Ordered Lipid Domains (Rafts):Implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Alterman M, Dobrowsky RT. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. J Lipid Res. 2005;46:1678–1691. doi: 10.1194/jlr.M500060-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Subbaiah PV, Monshizadegan H. Substrate specificity of human plasma lecithin-cholesterol acyltransferase towards molecular species of phosphatidylcholine in native plasma. Biochim Biophys Acta. 1988;963:445–455. doi: 10.1016/0005-2760(88)90313-x. [DOI] [PubMed] [Google Scholar]
- 33.Jonas A, Zorich NL, Kezdy KE, Trick WE. Reaction of discoidal complexes of apolipoprotein A-I and various phosphatidyl cholines with lecithin cholesterol acyltransferase. Interfacial effects. J Biol Chem. 1987;262:3969–3974. [PubMed] [Google Scholar]
- 34.Subbaiah PV, Liu M, Paltauf F. Role of sn-2 acyl group of phosphatidyl choline in determining the positional specificity of lecithin-cholesterol acyltransfersae. Biochemistry. 1994;33:13259–13266. doi: 10.1021/bi00249a012. [DOI] [PubMed] [Google Scholar]
- 35.Pownall HJ, Pao Q, Massey JB. Acyl chain and headgroup specificity of human plasma lecithin:cholesterol acyltransferase. Separation of matrix and molecular specificities. J Biol Chem. 1985;260:2146–2152. [PubMed] [Google Scholar]
- 36.Glomset JA. Physiological role of lecithin-cholesterol acyltransferase. Am J Clin Nutr. 1970;23:1129–1136. doi: 10.1093/ajcn/23.8.1129. [DOI] [PubMed] [Google Scholar]
- 37.Swell L, Field H, Treadwell CR. Correlation of arachidonic acid of serum cholesterol esters in different species with susceptibility to atherosclerosis. Proc Soc Exp Biol Med. 1960;104:325–328. doi: 10.3181/00379727-104-25823. [DOI] [PubMed] [Google Scholar]
- 38.Abdulla YH, Adams CWM, Morgan RS. Connective-tissue reactions to implantation of purified sterol esters, phosphoglycerides, glycerides and free fatty acids. J Path Bact. 1967;94:63–71. doi: 10.1002/path.1700940109. [DOI] [PubMed] [Google Scholar]
- 39.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, Evans JH, Freiberg J, Roddy P, Hannun YA, Chalfant CE. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 40.Barenholz Y. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell Biochem. 2004;37:167–215. doi: 10.1007/978-1-4757-5806-1_5. [DOI] [PubMed] [Google Scholar]
- 41.Slotte JP. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem Phys Lipids. 1999;102:13–27. doi: 10.1016/s0009-3084(99)00071-7. [DOI] [PubMed] [Google Scholar]
- 42.Barenholz Y. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Sub-Cellular Biochemistry. 2004;37:167–215. doi: 10.1007/978-1-4757-5806-1_5. [DOI] [PubMed] [Google Scholar]
- 43.Jain MK, Zakim D. The spontaneous incorporation of proteins into preformed bilayers. Biochim Biophys Acta. 1987;906:33–68. doi: 10.1016/0304-4157(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 44.Holopainen JM, Subramanian M, Kinnunen PKJ. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 45.Huwiler A, Johansen B, Skarstad A, Pfeilschifter J. Ceramide binds to the CaLB domain of cytosolic phospholipase A2 and facilitates its membrane docking and arachidonic acid release. FASEB J. 2001;15:7–9. doi: 10.1096/fj.00-0370fje. [DOI] [PubMed] [Google Scholar]
- 46.Thies F, Delachambre MC, Bentejac M, Lagarde M, Lecerf J. Unsaturated fatty acids esterified in 2-acyl-1- lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem. 1992;59:1110–1116. doi: 10.1111/j.1471-4159.1992.tb08353.x. [DOI] [PubMed] [Google Scholar]


