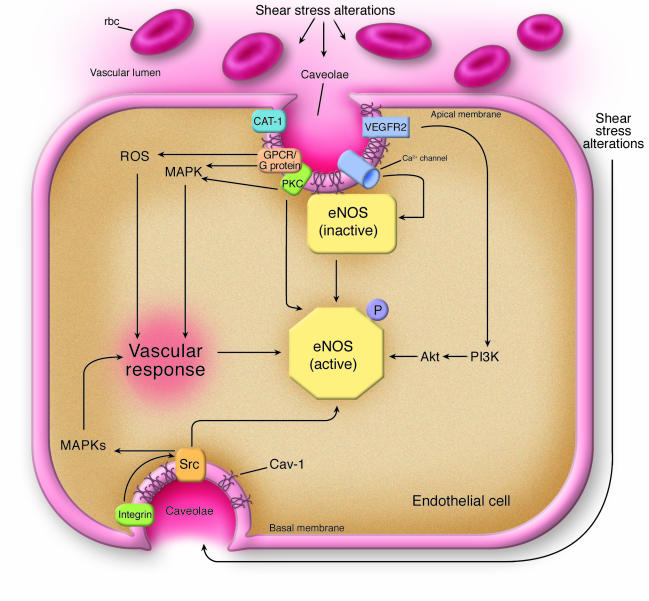
Figure 2. Cav-1–regulated signaling pathways in endothelial cells.
According to Yu et al. (20), caveolae and Cav-1 appear to be involved in some of the earliest steps associated with the detection of altered shear stress conditions in blood vessels. In addition, the vascular response (see Figure 1) is induced via caveolae and Cav-1, as demonstrated for several signaling pathways. Cav-1 plays important roles in regulating eNOS function. In unstimulated cells, eNOS is maintained in an inactive state through its association with Cav-1. Upon stimulation (e.g., shear stress), Cav-1 and caveolae may allow for the proper organization of various signal transduction pathways or organize the different regulatory proteins necessary for rapid and efficient eNOS activation. Calcium channels and the arginine transporter CAT-1 are localized to caveolae and may therefore allow for efficient eNOS activation (Ca2+ entry and dissociation from Cav-1 in the presence of calmodulin) and availability of substrate. Further activation is also possible via the PI3K and Akt signaling pathways, which enhance NO production following eNOS phosphorylation. Activation of the VEGFR2 signaling pathway is critical for PI3K/Akt activation, but other pathways appear to also mediate MAPK activation (ERK1/2 in particular). These pathways also involve G protein–coupled receptors (GPCRs) and G proteins. Shear stress is associated with increased oxidative stress conditions that lead to the production of ROS. Additionally, shear stress may affect EC interactions with extracellular matrix proteins. This may in turn activate integrin-mediated signaling pathways via caveolae. Src may also directly alter eNOS activity by inducing tyrosine phosphorylation (33). Note that only the major affected signal transduction pathways are shown.