Figure 4. Algorithm for HLA class II–Dsg3 peptide interaction in PV.
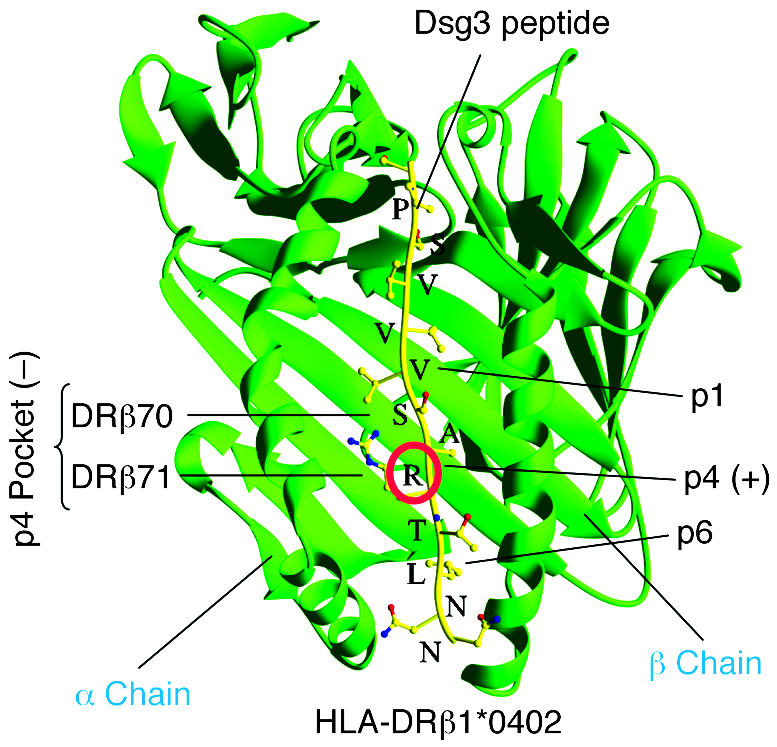
Shown is the physical interaction of DRβ1*0402 with a representative Dsg3 peptide. HLA-DRβ1*0402 differs from other HLA-DR4 molecules by the presence of a negative charge at amino acid residue 71 (DRβ71) of the β chain, which is a critical binding motif for T cell peptides. Several T cell epitopes of Dsg3, the major autoantigen of PV, have been identified that carry a positively charged amino acid (mostly lysine, K, or arginine, R) at relative position 4 (p4; red circle), which serves as an anchor motif to the negatively charged p4 pocket formed by residues DRβ70 and DRβ71 of DRβ1*0402. Thus DRβ1*0402 shapes the fine specificity of the T cell autoimmune response against a limited set of Dsg3 epitopes that fulfill the binding criteria of this specific HLA class II molecule.
