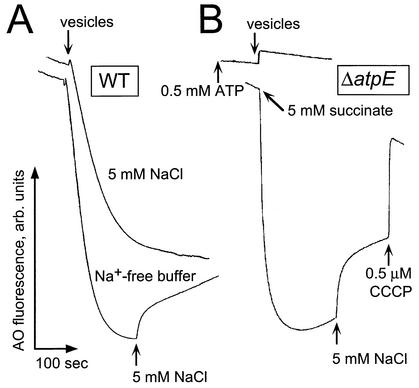
FIG. 1.
Formation of the ATP-dependent ΔpH in the inside-out subbacterial vesicles from V. cholerae. Aliquots of vesicles (300 μg of protein) were resuspended in 2.5 ml of the isolation buffer (see the text), with MOPS-Tris replaced by Tris-sulfate (pH 7.5 or 8.5 as indicated). The experimental buffer did not contain protease inhibitors and was supplemented with 0.5 μM acridine orange. The resulting quenching of acridine orange fluorescence was monitored in a Shimadzu RF-1501 spectrofluorometer with excitation at 492 nm and emission at 528 nm. (A) Wild-type (O395N1) V. cholerae. Tris-ATP (0.5 mM) was added to the reaction mixture prior to the addition of the vesicles. (B) ΔatpE mutant. Formation of the respiratory ΔpH was initiated by the addition of 5 mM succinate to the experimental mixture containing subbacterial vesicles. In the case of the ATP-dependent ΔpH, 0.5 mM Tris-ATP was added to the reaction mixture prior to the addition of the vesicles.