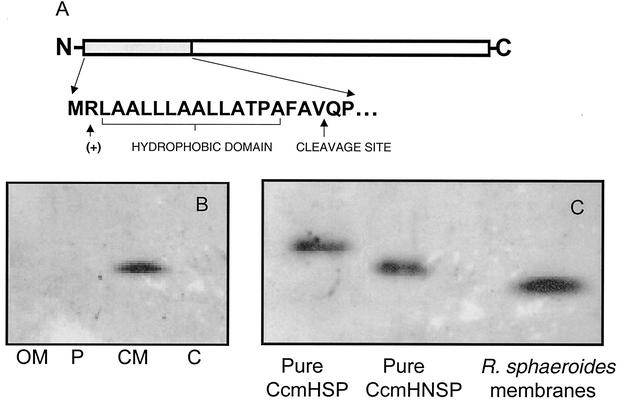
FIG. 4.
CcmH has a cleavable N-terminal signal sequence. (A) Amino acid similarity of the N-terminal region of the putative CcmH precursor protein to bacterial signal peptides, including a positively charged residue at position 2, a hydrophobic core of 16 to 17 residues, and a potential signal peptidase cleavage site. (B) Western blot analysis using CcmH antibody with outer membrane (OM), periplasm (P), cytoplasmic membrane (CM), and cytoplasmic (C) fractions from aerobically grown cells (25 μg of protein in each sample). (C) Results of a Western blot analysis using CcmH antibody to compare the relative mobility of a full-length His6-CcmH (CcmHSP), a CcmH protein which lacks the 19 N-terminal amino acids (CcmHNSP), and CcmH within the R. sphaeroides cytoplasmic membrane.