Abstract
Chlamydia pneumoniae is an obligate intracellular human pathogen that causes acute respiratory diseases such as pneumonia and bronchitis. Previous studies have established that C. pneumoniae can induce cytokines in mouse and/or human cells, but little information is available on the cytokine response of respiratory epithelial cells, a first line of infection. In this study, heparin treatment of C. pneumoniae significantly reduced its ability to induce interleukin 8 (IL-8) and tumor necrosis factor alpha (TNF-α) mRNA in human lung carcinoma cells, indicating that cytadherence is an important early stimulus for induction of proinflammatory mediators. Although the IL-8, gamma interferon, and TNF-α message was consistently induced by infection of A549 cells not treated with heparin, only an elevation of IL-8 protein was detected in A549 supernatants. A549 IL-β and IL-6 mRNA and supernatant protein profiles were not significantly changed by infection. Heat or UV inactivation of C. pneumoniae only partially reduced the cytokine response, and inhibition of C. pneumoniae protein or DNA synthesis did not affect its ability to induce cytokine gene expression. To prevent stress-induced cytokine release by the A549 cells, centrifugation was not utilized for infection experiments. These experiments establish the importance of cytadherence in cytokine release by cells of respiratory epithelial origin and suggest that further work in the area of cytokine mediators is warranted to gain valuable pathogenic and therapeutic insights.
The human pathogen Chlamydia pneumoniae is an obligate gram-negative intracellular bacterium with a biphasic life cycle. The metabolically inactive infectious elementary bodies (EBs) attach and enter the host cell, where they differentiate into the metabolically active reticulate bodies (RBs). The RBs replicate by binary fission within the expanding endosome, resulting in the development of intracellular inclusions. After a period of growth, these RBs reorganize into new EBs. With the subsequent lysis of the infected cell, the EBs are released and initiate the next replication cycle (25, 49).
C. pneumoniae causes acute respiratory infections such as bronchitis, pharyngitis, pneumonia, and sinusitis (25), and its role in chronic diseases such as asthma and reactive arthritis is an active area of investigation (15, 17, 31, 47). Recently, attention has been focused on the possible involvement of C. pneumoniae in atherosclerosis, based on seroepidemiological studies and direct detection of the organism in cardiovascular tissue (3, 14, 45).
Cytokines are important mediators in host defenses against bacterial and viral infections. Cytokines such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin 1 (IL-1), and lymphotoxin have been shown to inhibit chlamydial infection, and this inhibition is at least partially mediated by the activation of inducible nitric oxide synthase which releases nitric oxide (NO), an important antimicrobial and tumoricidal agent as well as a cell-signaling molecule (1, 4, 20, 30, 41, 43).
C. pneumoniae has also been shown to induce cytokines such as IL-1β, IL-6, IL-8, IL-12, TNF-α, IFN-γ, and intercellular adhesion molecule-1 in various systems such as human peripheral blood mononuclear cells, alveolar macrophages, and various mouse cell models (11, 16, 19, 22, 39). The possible relationship between C. pneumoniae and atherosclerosis led to many studies of the cytokine effects of infection on human endothelial cells and smooth muscle cells in which IL-6, IL-8, and monocyte chemotactic protein-1 were shown to be readily induced by infection and activation of NF-κB was shown to be a requirement for monocyte chemotactic protein-1 gene expression (6, 32, 33, 37, 40). C. pneumoniae infection can also stimulate the production of anti-inflammatory cytokines such as IL-10, which can down regulate the expression of major histocompatibility complex class I molecules (5, 16). Interestingly, C. pneumoniae-infected epithelial cells and peripheral blood mononuclear cells are resistant to apoptosis induced by chemicals or death receptors, and this resistance is at least partially due to IL-10 induction (12, 38). Thus, the overall effect of cytokine release by host cells following C. pneumoniae infection is likely to be determined by complex interactions between beneficial and detrimental cytokine actions.
Bronchial epithelial cells are a first line of defense during C. pneumoniae airway infection. It is known that epithelial cells at the mucosal surface are capable of secreting chemoattractants and proinflammatory cytokines, which are important mediators in both lung defense and inflammation, in response to bacterial infection. This suggests that epithelial cells can act as an early warning system for local immune and inflammatory cells (8, 27). For example, Jahn et al. showed that C. pneumoniae induced a time-dependent enhanced release of IL-8 and prostaglandin E2 and an increased expression of intercellular adhesion molecule-1 in primary human airway epithelial cells and the bronchial epithelial cell line BEAS-2B (21). However, little information exists on how early events such as cytadherence are involved in cytokine induction, and much more work on cytokine release following C. pneumoniae infections of respiratory epithelial cells are needed. Here we report on the importance of attachment on cytokine induction, thus providing evidence that early events in infection do play an important role in cytokine induction.
Centrifugation has been used for chlamydial culture and target cell infection experiments because it can increase inclusion formation (36, 46, 50). Nonetheless, in a recent study we found that the stress of centrifugation alone was able to up-regulate the expression of IL-1β mRNA in human lung epithelial carcinoma A549 cells without affecting the viability or proliferation of the cells (J. Yang, W. C. Hooper, D. J. Phillips, M. L. Tondella, and D. F. Talkington, Letter, Clin. Diag. Lab. Immunol. 9:1142-1143, 2002). In light of these findings, we did not utilize centrifugation to enhance C. pneumoniae infection of the A549 cells also used in these studies.
MATERIALS AND METHODS
C. pneumoniae culture.
C. pneumoniae CM-1 VR-1360 (American Type Culture Collection, Rockville, Md.) was grown in HEp-2 cells, and stocks were prepared as described previously (36, 39), except that HEp-2 cells were grown in Iscove's modified Dulbecco's medium with 10% fetal bovine serum (Gibco, Grand Island, N.Y.). Confluent monolayers were infected with C. pneumoniae by centrifugation (1,000 × g, 1 h), and the supernatant was replaced with culture medium supplemented with cycloheximide (1 μg/ml; Sigma, St. Louis, Mo.). At day 3 after infection, cells were centrifuged for 1 h at the same speed, and fresh medium was added. Cells were again centrifuged on days 4 and 5, and C. pneumoniae was harvested on day 7 by lysing the cells with a sonic dismembrator (Fisher Scientific). Host-cell debris was then removed by low-speed centrifugation (500 × g, 30 min at room temperature). C. pneumoniae was then enriched by centrifugation at 30,000 × g for 45 min and resuspended in Earle's minimum essential medium (EMEM) at 4°C. The inclusion forming units (IFUs) were determined by a 10-fold serial dilution of the stock culture inoculated in HEp-2 cells. C. pneumoniae was detected by using the Pathfinder chlamydia culture confirmation system (Bio-Rad, Hercules, Calif.) according to the manufacturer's instructions. This technique gave a titer of 107 to 108 IFU/ml.
Cell line and reagents.
A549 cells were grown in EMEM with 10% fetal bovine serum. These cells were derived in 1972 from a human lung carcinoma (American Type Culture Collection catalog no. CCL-185). The cells were free of mycoplasma as determined by a commercial Mycoplasma genus PCR primer set (Stratagene, La Jolla, Calif.). For C. pneumoniae infection, approximately 107 IFU of C. pneumoniae were directly inoculated to 106 A549 cells without centrifugation or cycloheximide supplementation, unless otherwise specified. For mock infection of A549 cells, HEp-2 cells were processed as described above in chlamydial culture but without C. pneumoniae inoculation. Cycloheximide, chloramphenicol, nalidixic acid, and heparin were all purchased from Sigma. Cycloheximide was prepared as a 1-mg/ml stock in EMEM and added to a final concentration of 1 μg/ml. Chloramphenicol was dissolved in absolute ethanol as a 10-mg/ml stock and added to a final concentration of 20 μg/ml. Nalidixic acid was dissolved in EMEM as a 5-mg/ml stock and added to a final concentration of 40 μg/ml. Heparin was dissolved in EMEM as a 5,000-U/ml stock.
Cytokine assays.
A549 cells (5 × 105) were seeded into a six-well plate and infected with C. pneumoniae for up to 24 h. The secreted cytokines in the culture supernatant were tested with enzyme-linked immunosorbent assays (ELISAs). An OptEIA human IL-1β set was used to detect IL-1β (sensitivity, 15.6 pg/ml) (BD Biosciences, San Diego, Calif.), and the DuoSet ELISA development systems were used to detect IL-6 (4.18 pg/ml), IL-8 (31.25 pg/ml), IFN-γ (15.6 pg/ml), and TNF-α (15.6 pg/ml) (R & D Systems, Minneapolis, Minn.). All assays were conducted by following the manufacturer's instructions. All experiments were conducted at least twice, and in each experiment every sample was run in duplicate.
Immunofluorescent microscopy.
To detect intracellular IL-1β, an indirect immunofluorescent method was used. Cells (104) were seeded into a 96-well plate. At 24 h postinfection, the culture medium was removed and 200 μl of ice-cold methanol was added to each well to fix and permeate the cells for 5 min at room temperature. The primary and secondary antibodies were diluted according to the manufacturer's suggestions. Specifically, after removing the methanol, 25 μl of mouse anti-human IL-1β antibody (1:250; BD Biosciences) was added to each well and incubated at 37°C for 30 min in the dark. Antibodies were removed, and wells were washed twice with phosphate-buffered saline (0.01 M, pH 7.2). Twenty-five microliters of fluorescein isothiocyanate-labeled goat anti-mouse antibody (1:1,000; BD Biosciences) was then added to each well and incubated at 37°C for another 30 min in the dark. After removal of the secondary antibody and washing with phosphate-buffered saline, mounting fluid (carbonate-buffered glycerol mounting fluid, pH 9.0) was added. The plate was observed by using an Olympus IX70 fluorescent microscope (Olympus Optical Co., Ltd., Tokyo, Japan), and the images were captured and analyzed by using the Optronics (Goleta, Calif.) program.
RNA preparation and quantification.
Total RNA was prepared from the cells by using the RNeasy mini kit (Qiagen, Valencia, Calif.). Briefly, medium was completely removed from the culture flasks, then 350 μl of RLT lysis buffer from the kit was added into each 25-cm2 flask to lyse the cells. After transferring the lysate to a 1.5-ml collection tube, 350 μl of 70% ethanol was added and mixed well by repeat pipetting. The mixture was again transferred to an RNeasy mini spin column and centrifuged for 15 s at 8,000 × g. The column was then washed with the RW1 and RPE buffers from the kit, and finally, the RNA was dissolved in 30 μl of DNase-free, RNase-free water. The quantity and purity of the RNA samples were determined by measuring the absorbance at 260 nm and the A260/A280 ratio on a spectrometer.
RT-PCR.
Ready-To-Go reverse transcription (RT)-PCR beads (Amersham Pharmacia Biotech Inc., Piscataway, N.J.) were used for the RT-PCR. Total RNA (0.5 μg) was added for each reaction. The primer sequences were as follows: IL-1β, 5′-AAA CAG ATG AAG TGC TCC TTC CAG G-3′ and 5′-TGG AGA ACA CCA CTT GTT GCT CCA-3′ (10); IL-6, 5′-GGC TGA AAA AGA TGG ATG CT-3′ and 5′-CCT GCT TCA CCA CCT TCT G-3′ (52); IL-8, 5′-AGA TAT TGC ACG GGA GAA-3′ and 5′-GAA ATA AAG GAG AAA CCA-3′; β-actin, 5′-CGG GAC CTG ACT GAC TAC-3′ and 5′-GAA GGA AGG CTG GAA GAG-3′ (28); IFN-γ, 5′-GAT GCT CTT CGA CCT TGA AAC AGC AT-3′ and 5′-ATG AAA TAT ACA AGT TAT ATC TTG GCT TTT-3′ (9); TNF-α, 5′-GAG TGA CAA GCC TGT AGC CCA TGT TGT AGC A-3′ and 5′-GCA ATG ATC CCA AAG TAG ACC TGC CCA GAC T-3′ (48). RT was carried out at 42°C for 30 min, and amplifications were carried out for 32 cycles of 30 s at 95°C, 30 s at 55°C, and 60 s at 72°C. The products were run on a 2% agarose gel and stained with ethidium bromide. The gels were then scanned in a fluorImager SI (Molecular Dynamics, Sunnyvale, Calif.) and quantified by using the ImageQuant, version 4.2A, program (Molecular Dynamics). The percentage of surface area under the peak of each band was normalized to the corresponding β-actin band. All experiments were conducted at least twice, and representative blots were chosen for figures.
Heparin treatment.
C. pneumoniae was incubated in EMEM only or with 10, 50, or 100 U of heparin/ml in EMEM for 1 h at room temperature and then was used to infect A549 cells.
Inactivation of C. pneumoniae.
For heat inactivation, C. pneumoniae was heated at 90°C for 10 min prior to infecting the A549 cells. For UV inactivation, C. pneumoniae was placed under a UV lamp (15 W at 30 cm) for 20 min (40).
Statistical analysis.
Statistical analysis was performed with Student's t test. A probability level of P < 0.05 was considered to be significant. Data are presented as means ± standard deviations.
RESULTS
C. pneumoniae infection induces proinflammatory cytokine gene expression in A549 cells.
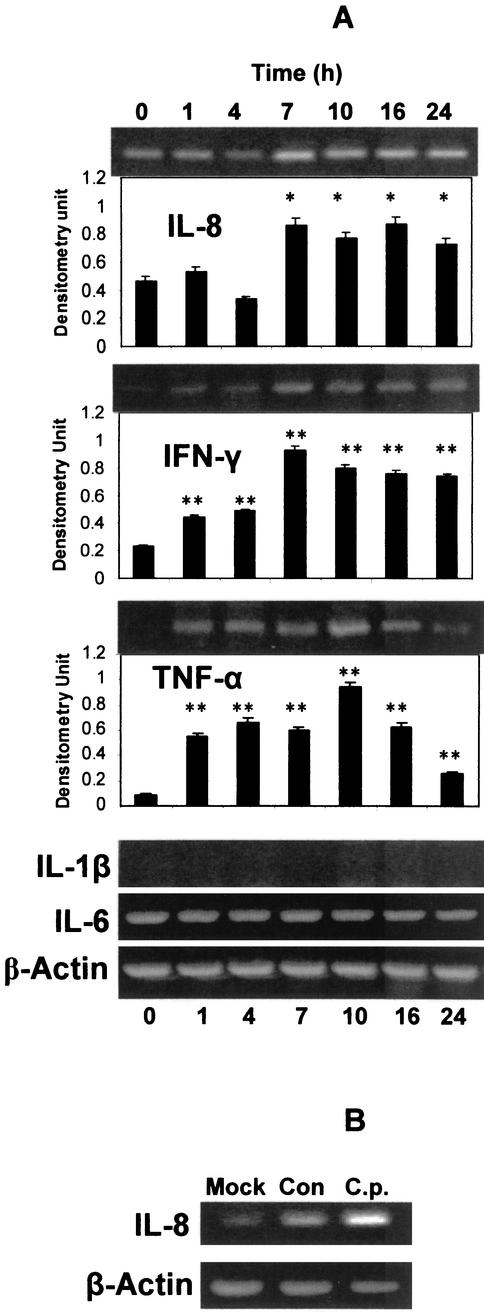
C. pneumoniae infection of A549 cells induced significant gene expression of several proinflammatory cytokines as detected with RT-PCR and confirmed by densitometry. Among the cytokines tested, IL-8, IFN-γ, and TNF-α mRNA levels were increased while IL-1β mRNA remained undetectable and IL-6 mRNA remained at the same constitutive level (Fig. 1A). IL-8 mRNA remained at constitutive levels following C. pneumoniae infection until significant elevations were present at 7, 10, 16, and 24 h after infection. IFN-γ mRNA levels followed a similar pattern but the baseline expression at time zero was relatively lower. The induction of TNF-α was transient; its mRNA appeared 1 h postinfection, peaked around 10 h, and while declining more than threefold over peak levels, remained significantly above baseline levels after 24 h postinfection. Internal control β-actin mRNA expression remained unchanged during the 24-h period of the experiments (Fig. 1A). Mock infection with processed HEp-2 cells induced some IL-8 gene expression in A549 cells, but it was significantly less than with bacteria (Fig. 1B).
FIG. 1.
Induction and expression of cytokine mRNA in A549 cells during C. pneumoniae infection. (A) A549 cells were inoculated with C. pneumoniae for 1 to 24 h, and then cellular levels of IL-8, IFN-γ, TNF-α, IL-1β, and IL-6 were measured by RT-PCR. β-Actin was used as an internal control. The RT-PCR products were run on agarose gels, stained with ethidium bromide, and visualized with UV. Densitometric quantifications for IL-8, IFN-γ, and TNF-α are shown below the agarose gel sections for those cytokines after normalization to β-actin. The densitometric values were compared to those at time zero and analyzed by Student's t test, *, P < 0.05; **, P < 0.01. Error bars represent standard deviations. (B) HEp-2 cell mock experiment. To make sure HEp-2 cell debris did not induce experimental variation, IL-8 mRNA levels were determined 24 h after A549 cells were treated with HEp-2 cell debris (Mock), diluent only (Con), or C. pneumoniae derived from HEp-2 culture (C.p.) incubated with A549 cells. Although less IL-8 message was induced in the Mock versus diluent (Con) group, the difference was not significant compared to the much greater magnitude of IL-8 mRNA induced by infection (C.p. group).
IL-8, but not IFN-γ nor TNF-α, proteins are detected in A549 cell culture medium.
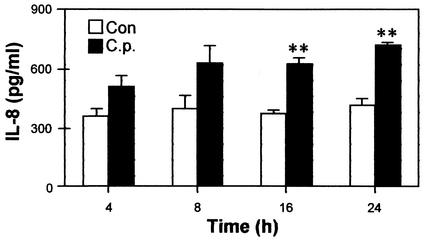
Because IL-8, IFN-γ, and TNF-α mRNA levels were increased after C. pneumoniae infection, we investigated the levels of protein expression for these cytokines. Cell culture supernatants were collected at specific time points after infection, and analyzed by ELISA. As shown in Fig. 2, IL-8 protein was steadily expressed in control A549 cells over a 24-h period. In comparison, infected A549 cells released increased levels of protein at all time points, and levels were significantly above the baseline at 16 and 24 h postinfection. In contrast, the protein levels for IFN-γ and TNF-α (and IL-1β and IL-6 proteins as well) were undetectable in both the control cells and infected cells (data not shown).
FIG. 2.
Increased IL-8 secretion in A549 cells in response to C. pneumoniae infection. Each time point was measured in triplicate; error bars represent standard deviations. A549 cells were either untreated (controls [Con]) or inoculated with C. pneumoniae (C.p.). At the time points specified postinfection, culture supernatants were collected and secreted IL-8 was measured by ELISA. Each pair of data at the same time point was analyzed by Student's t test. **, P < 0.01.
Since IL-1β protein has a precursor form that is predominantly intracellular (2, 18), we used an indirect immunofluorescent method to determine if the precursor IL-1β protein was present in C. pneumoniae-infected cells. Consistent with the mRNA results, there was no precursor IL-1β detected intracellularly (data not shown).
C. pneumoniae cytadherence is important for cytokine induction.
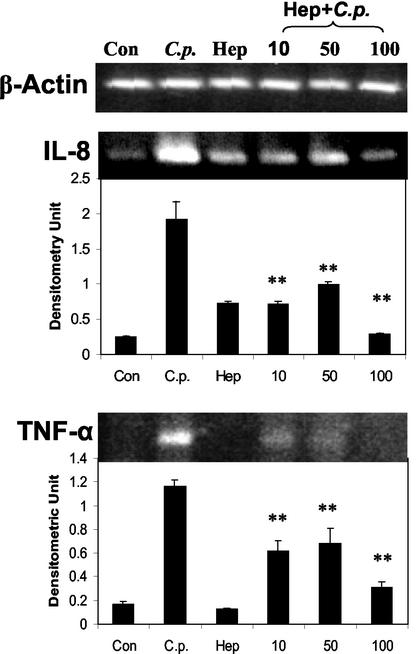
Recently, it was reported that the heparan sulfate-like glycosaminoglycan (GAG) is a cellular receptor for C. pneumoniae. Heparin treatment of C. pneumoniae inhibited attachment of bacteria to human epithelial cells, and this reduction in infectivity was shown to be the result of heparin binding to the bacteria (51). Using this observation, we examined the role of cytadherence of C. pneumoniae to A549 cells in the induction of cytokine gene expression. C. pneumoniae was either incubated with heparin in EMEM at different concentrations (10, 50, and 100 U/ml) for 1 h or left at room temperature in EMEM for 1 h before being used to infect A549 cells. At 10 h postinfection, total RNA was extracted and the expression of the IL-8 and TNF-α message was determined by RT-PCR. As shown in Fig. 3, uninfected control cells weakly expressed IL-8 mRNA, while C. pneumoniae in EMEM alone effectively induced IL-8 mRNA expression. However, heparin-treated C. pneumoniae showed a significantly decreased ability to induce IL-8 expression; 100 U of heparin/ml almost completely blocked induction of IL-8 mRNA by C. pneumoniae, whereas concentrations of 50 or 10 U/ml were less effective but still significantly blocked induction (Fig. 3). RT-PCR analysis for TNF-α showed a similar pattern, as 100 U of heparin/ml more-effectively inhibited C. pneumoniae-induced expression (Fig. 3). This shows that the bacterial cells themselves were responsible for cytokine induction and not soluble mediators, such as heat shock proteins, reported to be released as by-products of chlamydia culture in HEp-2 cells (7). Thus, cytadherence appears to be important for induction of cytokine expression by C. pneumoniae.
FIG. 3.
Cytadherence is important for C. pneumoniae infection-induced cytokine gene expression. A549 cells were either left untreated (Con), infected with C. pneumoniae (C.p.), or incubated with 100 U of heparin (Hep)/ml. For heparin treatment of C. pneumoniae, the bacterium was incubated with heparin (Hep+C.p.) at concentrations of 10, 50, and 100 U/ml for 1 h; the mixtures were then used to infect A549 cells. Ten hours later, the total RNA was extracted and cellular levels of IL-8 and TNF-α were measured by RT-PCR. β-Actin was used as an internal control. Densitometric quantification of the electrophoretograms for IL-8 and TNF-α RT-PCR products are shown below the gel pictures and normalized to β-actin. The densitometric values of Hep+C.p. were compared to those of C.p. and analyzed by Student's t test. **, P < 0.01.
Heat or UV inactivation of C. pneumoniae decreases but does not abolish the induction of cytokine gene expression in A549 cells.
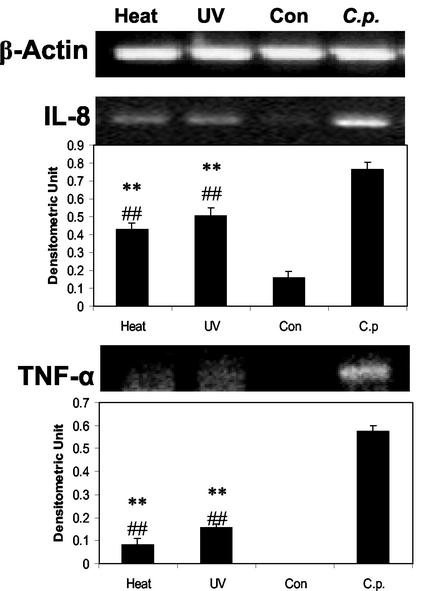
Although it has been reported that sonicated C. pneumoniae can stimulate cytokine production in human blood mononuclear cells (35), others have reported that live C. pneumoniae is required for IL-6 production in human bronchial smooth muscle cells (40). To determine if live and inactivated C. pneumoniae have similar cytokine inductive capacities in A549 cells, both heat and UV inactivation were separately employed before the addition of bacteria to A549 cells. Ten hours later, the expression of IL-8 or TNF-α mRNA was tested by RT-PCR. Although IL-8 expression in inactivated-C. pneumoniae-treated cells was significantly lower than that induced by live-C. pneumoniae-infected A549 cells, as shown in Fig. 4, IL-8 expression in A549 cells mixed with either heat- or UV-inactivated C. pneumoniae was significantly higher than that of the uninfected A549 control cells, indicating that live bacteria are not necessary for cytokine induction. TNF-α expression showed a similar pattern, but the magnitude of difference between killed and live bacteria was much more pronounced (Fig. 4).
FIG. 4.
Effects of heat or UV inactivation of C. pneumoniae on its ability to induce cytokine gene expression in A549 cells. A549 cells were left untreated (Con) or infected with C. pneumoniae (C.p.), heat-treated C. pneumoniae (Heat), or UV-treated C. pneumoniae (UV). Ten hours later, the total RNA was extracted and cellular levels of IL-8 and TNF-α were measured by RT-PCR. β-Actin was used as an internal control. Densitometric quantifications of the electrophoretograms after normalization to β-actin appear below the gel pictures of IL-8 and TNF-α RT-PCR products. The densitometric values of heat or UV treatment were compared to those of C. pneumoniae and analyzed by Student's t test (**, P < 0.01). These values were also compared to those of the controls and analyzed by Student's t test (##, P < 0.01).
C. pneumoniae protein synthesis and DNA synthesis are not required in the induction of cytokine gene expression in A549 cells.
To determine if bacterial protein or DNA synthesis was necessary to stimulate cytokine induction of A549 cells, we conducted experiments utilizing 20 μg of chloramphenicol (a bacterial protein synthesis inhibitor)/ml or 40 μg of nalidixic acid (a bacterial DNA synthesis inhibitor)/ml. When A549 cells were infected with C. pneumoniae for 2 h (to allow attachment to occur) and then treated with chloramphenicol or nalidixic acid, elevations in expression of IL-8 or TNF-α mRNA 8 h after treatment were not significantly different from those seen in infected A549 cells not treated with either chemical (data not shown). Treatment of A549 cells with chloramphenicol or nalidixic acid 2 h before, concurrently with, or 2 h after bacterial infection gave similar results. These data lead to the conclusion that early events in C. pneumoniae infection (such as attachment or penetration into host cells), rather than later events in C. pneumoniae replication cycle (such as DNA or protein syntheses) are primarily responsible for the induction of host cell proinflammatory cytokine gene expression.
Furthermore, A549 cells pretreated with cycloheximide to inhibit protein synthesis released significantly less IL-8 after bacterial infection, suggesting that de novo mediator synthesis rather than release of the preformed mediator was primarily responsible for IL-8 expression following infection (data not shown).
DISCUSSION
The induction of proinflammatory cytokines by C. pneumoniae infection may play an important role in the pathogenesis of C. pneumoniae infection, not only in acute respiratory infections but also in chronic infections and atherosclerosis (13, 23, 39, 41, 42). In this study, we found that C. pneumoniae infection of A549 respiratory carcinoma cells induced gene expression of the proinflammatory cytokines IL-8, IFN-γ, and TNF-α. In addition, IL-8 protein levels were increased in the cell culture medium following infection with C. pneumoniae. IL-8, a potent chemoattractant and activator of neutrophils, monocytes, and T lymphocytes, is the major contributor for the neutrophil influx to the lung during bacterial infections (24, 34).
The attachment of C. pneumoniae to the host cell is an important initial step in the establishment of infection. Building upon Wuppermann et al.'s finding that heparan sulfate-like GAG is a cellular receptor for C. pneumoniae and that preincubation of C. pneumoniae with heparin reduced the infectivity of the bacterium (51), we showed that this same treatment significantly reduced the ability of C. pneumoniae to induce cytokine gene expression (Fig. 3). This is the first direct evidence that cytadherence of C. pneumoniae to cells is very important in the induction of host cytokine responses. Because soluble mediators such as chlamydia heat shock protein 60 released into HEp-2 supernatants during chlamydia culture are capable of inducing cytokines independently (6) and may play an independent biological role in pathogenesis (23), it is critical to have evidence that the bacteria themselves can induce cytokines. We also found that C. pneumoniae protein or DNA synthesis is not required for cytokine induction, leading to the conclusion that earlier events during C. pneumoniae infection, e.g., attachment, rather than later events (e.g., escape from intracellular lysosomal destruction) in the replication cycle, may be responsible for triggering the host cell mediator responses.
In the A549 respiratory target cell system employed here, heat or UV inactivation of C. pneumoniae significantly reduced cytokine gene expression (Fig. 4). However, such inactivation did not completely abolish its ability to induce cytokine expression, and inactivated organisms could still stimulate a significant expression of cytokines. The interaction between the host receptors and C. pneumoniae surface molecules may be dependent on proper structural conformation of cell surface components in live bacteria to achieve maximum cytokine induction; alternately, heat or UV treatment might disrupt membrane structure or surface molecule conformation, thereby decreasing the ability of C. pneumoniae to induce host cytokine responses regardless of viability. These data support and extend earlier findings which suggest that live C. pneumoniae can provide the strongest stimulus for mediator release but that killed bacteria can also stimulate a lesser, though significant, release in cells different from those used here but also with a respiratory airway origin (21).
The observation that cytadherence is important for cytokine induction provides further evidence that the interaction between GAG receptors on the host cell membrane and GAG-binding molecules on the C. pneumoniae surface is an important one. There may be other receptors for C. pneumoniae on host cells, and there may be more than one type of surface molecule on C. pneumoniae involved in the interaction with host receptors (50). However, the presence of ligands on dead or living bacterial cells for cytokine receptors offers the attractive experimental strategy of trying to selectively block these ligands therapeutically in the respiratory tract should there be benefit to the host in doing so. For example, there is evidence that inflammation induced by cytokines in atherosclerotic plaques is often detrimental (7), but the overall effect of such inflammation in the respiratory tract is not currently understood. Others have speculated that the attachment of the pathogen may be sufficient to initiate an epithelial inflammatory response and that bacterial uptake might not be required for this process (21). Our results with heat- and UV-killed bacteria support this possibility.
In many in vitro studies of C. pneumoniae, a centrifugation step is employed to facilitate the infection. However, centrifugation stress has been known to affect certain cellular functions, such as cell proliferation or gene expression (26, 29, 44), and centrifugation alone can up-regulate IL-1β gene expression in A549 cells (Yang et al., letter). Therefore in our studies, A549 cells were infected with C. pneumoniae without centrifugation.
In this study we have determined that blocking bacterial adherence significantly reduces cytokine induction in A549 cells, suggesting that early events during infection are clearly important for cytokine induction. The data presented in this study provide a better understanding of cytokine release following C. pneumoniae infection of an important first line of defense, the respiratory epithelial cell. Continued studies of cytokine induction may provide insight into new therapeutic strategies for limiting the pathology caused by C. pneumoniae infection.
Acknowledgments
We gratefully thank the colleagues of the Respiratory Diseases Laboratory Section at the Centers for Disease Control and Prevention for help and advice during the preparation of the manuscript, especially S. Schwartz, L. Thacker, K. Cowley, and R. Benson for technical assistance and valuable suggestions.
J. Yang was supported by the American Society for Microbiology/National Center for Infectious Diseases Postdoctoral Research Associate Program.
Editor: J. D. Clements
REFERENCES
- 1.Airenne, S., H. M. Surcel, A. Bloigu, K. Laitinen, P. Saikku, and A. Laurila. 2000. The resistance of human monocyte-derived macrophages to Chlamydia pneumoniae infection is enhanced by interferon-gamma. APMIS 108:139-144. [DOI] [PubMed] [Google Scholar]
- 2.Black, R. A., S. R. Kronhcim, M. Cantrell, M. C. Deeley, D. J. March, K. S. Prickett, J. Wignall, P. J. Conlon, D. Cosman, T. P. Hopp, and D. Y. Mochizuki. 1988. Generation of biologically active interleukin-1β by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 263:9437-9442. [PubMed] [Google Scholar]
- 3.Campell, L. A., E. R. O'Brien, A. L. Cappuccio, C. C. Kuo, S. P. Wang, D. Stewart, D. L. Patton, P. K. Cummings, and J. T. Grayston. 1995. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J. Infect. Dis. 172:585-588. [DOI] [PubMed] [Google Scholar]
- 4.Carlin, J. M., and J. B. Weller. 1995. Potentiation of interferon-mediated inhibition of Chlamydia infection by interleukin-1 in human macrophage cultures. Infect. Immun. 63:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspar-Bauguil, S., B. Puissant, D. Nazzal, J.-C. Lefevre, M. Thomsen, R. Salvayre, and H. Benoist. 2000. Chlamydia pneumoniae induces interleukin-10 production that down-regulates major histocompatibility complex class I expression. J. Infect. Dis. 182:1394-1401. [DOI] [PubMed] [Google Scholar]
- 6.Dechend, R., M. Maass, J. Gieffers, R. Dietz, C. Scheidereit, A. Leutz, and D. C. Gulba. 1999. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-κB and induces tissue factor and PAI-1 expression. Circulation 100:1369-1373. [DOI] [PubMed] [Google Scholar]
- 7.Donath, B., C. Fischer, S. Page, S. Prebeck, N. Jilg, M. Weber, C. da Costa, D. Neumeier, T. Miethke, and K. Brand. 2002. Chlamydia pneumoniae activates IKK/IkappaB-mediated signaling, which is inhibited by 4-HNE following primary exposure. Atherosclerosis 165:79-88. [DOI] [PubMed] [Google Scholar]
- 8.Eckmann, L., M. F. Lagnoff, and J. Fierer. 1995. Intestinal epithelial cells as watchdogs for the natural immune system. Trends Microbiol. 3:118-120. [DOI] [PubMed] [Google Scholar]
- 9.Fenton, M. J., M. W. Vermeulen, S. Kim, M. Burdick, R. M. Strieter, and H. Kornfeld. 1997. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect. Immun. 65:5149-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan, X., and B. Bonavida. 1999. Preferential induction of TNF-α and IL-1β and inhibition of IL-10 secretion by human peripheral blood monocytes by synthetic Aza-alkyl lysophopholipids. Cell. Immunol. 193:125-133. [DOI] [PubMed] [Google Scholar]
- 11.Geng, Y., K. Berencsi, Z. Gyulai, T. Valyi-Nagy, E. Gonczol, and G. Trinchieri. 2000. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect. Immun. 68:2245-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng, Y., R. B. Shane, K. Berencsi, E. Gonczol, M. H. Zaki, D. J. Margolis, G. Trinchieri, and A. H. Rook. 2000. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J. Immunol. 164:5522-5529. [DOI] [PubMed] [Google Scholar]
- 13.Gerard, H. C., Z. Wang, J. A. Whittum-Hudson, H. El-Gabalawy, R. Goldbach-Mansky, T. Bardin, H. R. Schumacher, and A. P. Hudson. 2002. Cytokine and chemokine mRNA produced in synovial tissue chronically infected with Chlamydia trachomatis and C. pneumoniae. J. Rheumatol. 29:1827-1835. [PubMed] [Google Scholar]
- 14.Gibbs, R. G., N. Carey, and A. H. Davies. 1998. Chlamydia pneumoniae and vascular disease. Br. J. Surg. 85:1191-1197. [DOI] [PubMed] [Google Scholar]
- 15.Hahn, D. L., and R. McDonald. 1998. Can acute Chlamydia pneumoinae respiratory infection initiate chronic asthma? Ann. Allergy Asthma Immunol. 81:339-344. [DOI] [PubMed] [Google Scholar]
- 16.Halme, S., J. Latvala, R. Karttunen, I. Palatsi, P. Saikku, and H.-M. Surcel. 2000. Cell-mediated innune response during primary Chlamydia pneumoniae infection. Infect. Immun. 68:7156-7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannu, T., M. Puolakkainen, and M. Leirisalo-Repo. 1999. Chlamydia pneumoniae as a triggering infection in reactive arthritis. Rheumatology 38:411-444. [DOI] [PubMed] [Google Scholar]
- 18.Hazuda, D. J., J. Strickler, P. Simon, and P. R. Young. 1991. Structure-function mapping of interleukin 1 precursors. Cleavage leads to a conformational change in the mature protein. J. Biol. Chem. 266:7081-7086. [PubMed] [Google Scholar]
- 19.Heinemann, M., M. Susa, U. Simnacher, R. Marre, and A. Essig. 1995. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect. Immun. 64:4872-4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igietseme, J. U., I. M. Uriri, M. Chow, E. Abe, and R. G. Rank. 1997. Inhibition of intracellular multiplication of human strains of Chlamydia trachomatis by nitric oxide. Biochem. Biophys. Res. Commun. 232:595-601. [DOI] [PubMed] [Google Scholar]
- 21.Jahn, H.-U., M. Krull, F. N. Wuppermann, A. C. Klucken, S. Rosseau, J. Seybold, J. H. Hegemann, C. A. Jantos, and N. Suttorp. 2000. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J. Infect. Dis. 182:1678-1687. [DOI] [PubMed] [Google Scholar]
- 22.Kaukoranta-Tolvanen, S.-S. E., A.-M. Teppo, K. Laitinen, P. Saikku, K. Linnavuori, and M. Leinonen. 1996. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb. Pathog. 21:215-221. [DOI] [PubMed] [Google Scholar]
- 23.Kol, A., G. K. Sokova, A. H. Lichtman, P. Libby. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300-307. [DOI] [PubMed] [Google Scholar]
- 24.Kunkel, S. L., T. J. Standiford, K. Kasahara, and R. M. Strieter. 1991. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp. Lung. Res. 17:17-23. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, C. C., L. A. Jackson, L. A. Campbell, and J. T. Grayston. 1995. Chlamydia pneumoniae (TWAR). Clin. Microbiol. Rev. 8:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, D. H., J. C. Park, and H. Suh. 2001. Effect of centrifugal force on cellular activity of osteoblastic MC3T3-E1 cells in vitro. Yonsei Med. J. 42:405-410. [DOI] [PubMed] [Google Scholar]
- 27.Levine, S. J. 1995. Bronchial epithelial cell-cytokine interactions in airway inflammation. J. Investig. Med. 43:241-249. [PubMed] [Google Scholar]
- 28.Liechty, K. W., T. M. Crombleholme, D. L. Cass, B. Martin, and N. S. Adzick. 1998. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J. Surg. Res. 77:80-84. [DOI] [PubMed] [Google Scholar]
- 29.Maccarrone, M., M. Bari, and A. Finazzi Agro. 1999. Lipid peroxidation and polyamine metabolism in K562 cells subjected to altered gravity. J. Gravit. Physiol. 6:25-26. [PubMed] [Google Scholar]
- 30.Matsushima, H., M. Shirai, K. Ouchi, K. Yamashita, T. Kakutani, S. Furukawa, and T. Nakazawa. 1999. Lymphotoxin inhibits Chlamydia pneumoniae growth in HEp-2 cells. Infect. Immun. 67:3175-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meylby, K. K., T. K. Kvien, A. Glennas, and G. Anestad. 1999. Chlamydia pneumoniae as a trigger of reactive arthritis. Scand. J. Infect. Dis. 31:327-328. [DOI] [PubMed] [Google Scholar]
- 32.Molestina, R. E., R. D. Miller, A. B. Lentsch, J. A. Ramirez, and J. T. Summersgill. 2000. Requirement for NF-κB in transcriptional activation of monocyte chemotactic protein 1 by Chlamydia pneumoniae in human endothelial cells. Infect. Immun. 68:4282-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molestina, R. E., R. D. Miller, J. A. Ramirez, and J. T. Summersgill. 1999. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect. Immun. 67:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukaida, N., A. Harada, and K. Matsushima. 1998. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 9:9-23. [DOI] [PubMed] [Google Scholar]
- 35.Netea, M. G., C. H. Selzman, B. J. Kullberg, J. M. D. Galama, A. Weinberg, A. F. H. Stalenhoef, J. W. M. Van der Meer, and C. A. Dinarello. 2000. Acellular components of Chlamydia pneumoniae stimulate cytokine production in human blood mononuclear cells. Eur. J. Immunol. 30:541-549. [DOI] [PubMed] [Google Scholar]
- 36.Pruckler, J. M., N. Masse, V. A. Stevens, L. Gang, Y. Yang, E. R. Zell, S. F. Dowell, and B. S. Fields. 1999. Optimizing culture of Chlamydia pneumoniae by using multiple centrifugations. J. Clin. Microbiol. 37:3399-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinn, T. C., and C. A. Gaydos. 1999. In vitro infection and pathogenesis of Chlamydia pneumoniae in endovascular cells. Am. Heart J. 138:S507-S511. [DOI] [PubMed] [Google Scholar]
- 38.Rajalingam, K., H. Al-Younes, A. Muller, T. F. Meyer, A. J. Szczepek, and T. Rudel. 2001. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 69:7880-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redecke, V., K. Dalhoff, S. Bohnet, J. Braun, and M. Maass. 1998. Interaction of Chlamydia pneumoniae and human alveolar macrophages: infection and inflammatory response. Am. J. Respir. Cell Mol. Biol. 19:721-727. [DOI] [PubMed] [Google Scholar]
- 40.Rodel, J., M. Woytas, A. Groh, K.-H. Schmidt, M. Hartmann, M. Lehmann, and E. Straube. 2000. Production of basic fibroblast growth factor and interleukin-6 by human smooth muscle cells following infection with Chlamydia pneumoniae. Infect. Immun. 68:3635-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rottenberg, M. E., A. Gigliotti Rothfuchs, D. Gigliotti, M. Ceausu, C. Une, V. Levitsky, and H. Wigzell. 2000. Regulation and role of IFN-gamma in the innate resistance to infection with Chlamydia pneumoniae. J. Immunol. 164:4812-4818. [DOI] [PubMed] [Google Scholar]
- 42.Rottenberg, M. E., A. Gigliotti-Rothfuchs, and H. Wigzell. 2002. The role of IFN-gamma in the outcome of chlamydial infection. Curr. Opin. Immunol. 14:444-451. [DOI] [PubMed] [Google Scholar]
- 43.Summersgill, J. T., N. N. Sahney, C. A. Gaydos, T. S. Quinn, and J. A. Ramirez. 1995. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect. Immun. 63:2801-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theilig, C., A. Bernd, G. Leyhausen, R. Kaufmann, and W. Geurtsen. 2001. Effects of mechanical force on primary human fibroblasts derived from the gingiva and the periodontal ligament. J. Dent. Res. 80:1777-1800. [DOI] [PubMed] [Google Scholar]
- 45.Thom, D. H., S. P. Wang, J. T. Grayston, D. S. Siscovick, D. K. Stewart, R. A. Kronmal, and N. S. Weiss. 1991. Chlamydia pneumoniae strain TWAR antibody and angiographically demonstrated coronary artery disease. Arterioscler. Thromb. 11:547-551. [DOI] [PubMed] [Google Scholar]
- 46.Tjhie, J. H. T., R. Roosendaal, D. M. MacLaren, and C. M. Yandenbroucke-Grauls. 1997. Improvement of growth of Chlamydia pneumoniae on HEp-2 cells by pretreatment with polyethylene glycol in combination with additional centrifugation and extension of culture time. J. Clin. Microbiol. 35:1883-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Hertzen, L. C. 1998. Chlamydia pneumoniae and its role in chronic obstructive pulmonary diseases. Ann. Med. 30:27-37. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Y., and S. W. Walsh. 1996. TNF alpha concentrations and mRNA expression are increased in preeclampitc placentas. J. Reprod. Immunol. 32:157-169. [DOI] [PubMed] [Google Scholar]
- 49.Ward, M. E. 1995. The immunobiology and immunopathology of chlamydial infections. APMIS 103:769-796. [DOI] [PubMed] [Google Scholar]
- 50.Wong, K. H., S. K. Skelton, and Y. K. Chan. 1992. Efficient culture of Chlamydia pneumoniae with cell lines derived from the human respiratory tract. J. Clin. Microbiol. 30:1625-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wuppermann, F. N., J. H. Hegemann, and C. A. Jantos. 2001. Heparan sulfate-like glycosaminoglycan is a cellular receptor for Chlamydia pneumoniae. J. Infect. Dis. 184:181-187. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida, Y., M. Maruyama, T. Fujita, N. Arai, R. Hayashi, J. Araya, S. Matsui, N. Yamashita, E. Sugiyama, and M. Kobayashi. 1999. Reactive oxygen intermediates stimulate interleukin-6 production in human bronchial epithelial cells. Am. J. Physiol. 276:L900-L908. [DOI] [PubMed] [Google Scholar]