Abstract
Transmission blocking immunity induced by microgamete vaccination is fully effective for at least 12 months. Passive transfer of immune T cells reduced transmission of a subsequent infection by 95%, the effect being partly due to a significant reduction in numbers of circulating gametocytes during the infection. This immunity was apparently independent of specific antibody, though these were produced within a few days after challenge infection and was mediated by a T cell of the GK1.5+, Ly 2.2 phenotype. Immune serum and immune T cells, administered together, showed a strong additive effect and blocked transmission completely.
Full text
PDF

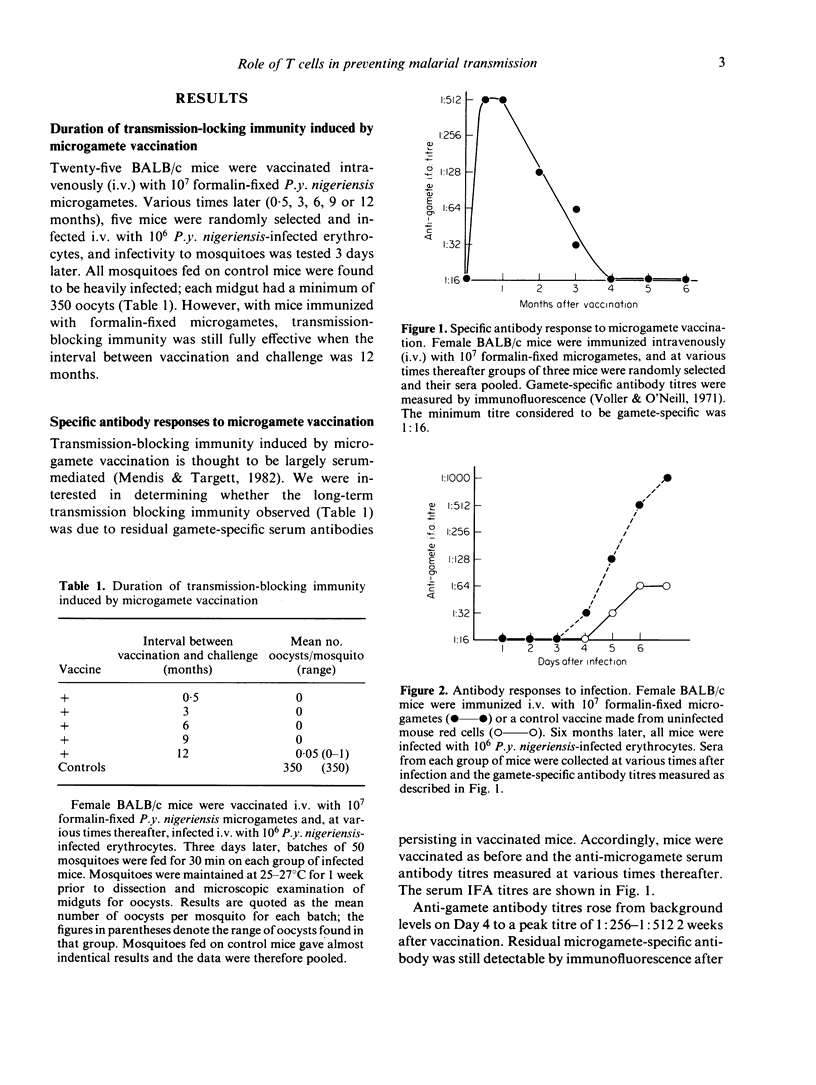
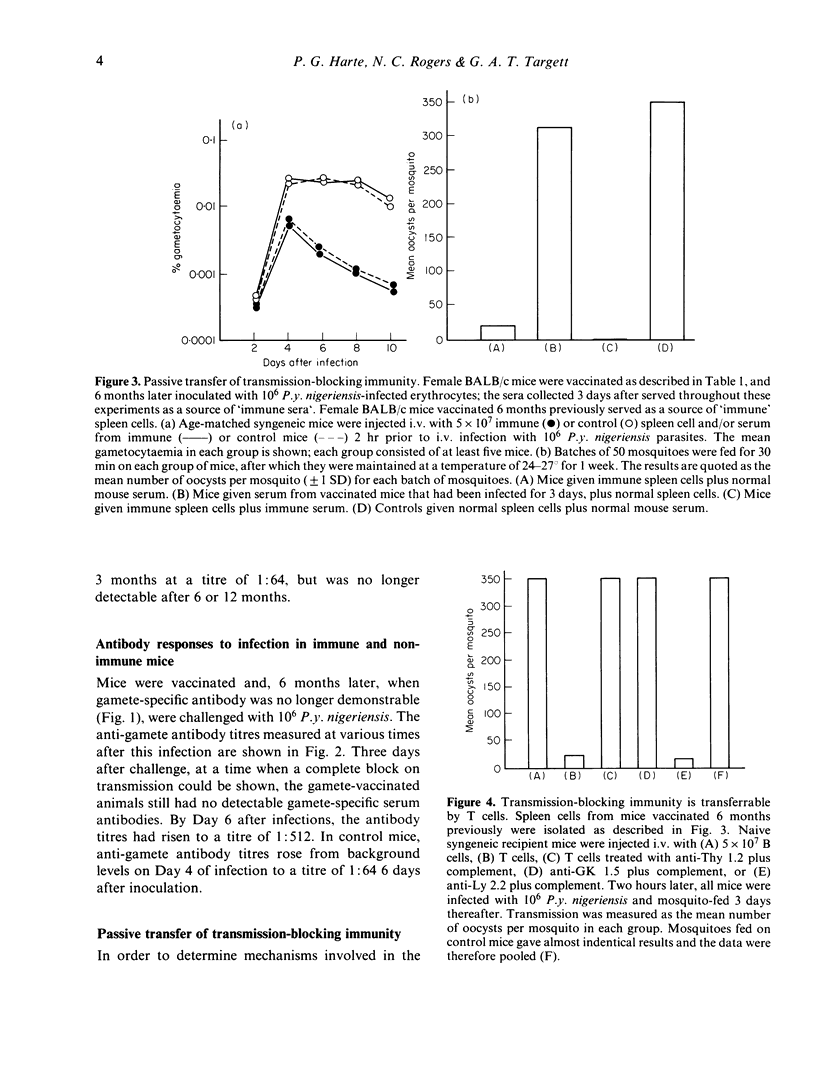



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazin H., Malet F. The metabolism of different immunoglobulin classes in irradiated mice. Immunology. 1969 Sep;17(3):345–365. [PMC free article] [PubMed] [Google Scholar]
- Carter R., Nijhout M. M. Control of gamete formation (exflagellation) in malaria parasites. Science. 1977 Jan 28;195(4276):407–409. doi: 10.1126/science.12566. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Richmond J. E., Wills E. J., Allison A. C. Immunity to intra-erythrocytic protoza. Lancet. 1975 Dec 6;2(7945):1128–1129. doi: 10.1016/s0140-6736(75)91010-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Wills E. J., Richmond J. E., Allison A. C. Suppression of babesiosis in BCG-infected mice and its correlation with tumor inhibition. Infect Immun. 1977 Aug;17(2):430–438. doi: 10.1128/iai.17.2.430-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., de Souza J. B., Playfair J. H. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980 Oct;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- EYLES D. E. Studies on Plasmodium gallinaceum. II. Factors in the blood of the vertebrate host influencing mosquito infection. Am J Hyg. 1952 Mar;55(2):276–290. doi: 10.1093/oxfordjournals.aje.a119521. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. Parasitic protozoa of the blood of rodents. I. The life-cycle and zoogeography of Plasmodium berghei nigeriensis subsp. nov. Ann Trop Med Parasitol. 1973 Sep;67(3):261–277. doi: 10.1080/00034983.1973.11686887. [DOI] [PubMed] [Google Scholar]
- Knight A., Sinden R. E. The purification of gametocytes of Plasmodium falciparum and P. yoelii nigeriensis by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1982;76(4):503–509. doi: 10.1016/0035-9203(82)90150-x. [DOI] [PubMed] [Google Scholar]
- Lee S. K., Singh J., Taylor R. B. Subclasses of T cells with different sensitivities to cytotoxic antibody in the presence of anesthetics. Eur J Immunol. 1975 Apr;5(4):259–262. doi: 10.1002/eji.1830050408. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Mendis K. N., Targett G. A. Immunisation against gametes and asexual erythrocytic stages of a rodent malaria parasite. Nature. 1979 Feb 1;277(5695):389–391. doi: 10.1038/277389a0. [DOI] [PubMed] [Google Scholar]
- Mendis K. N., Targett G. A. Vaccination to prevent transmission of Plasmodium yoelii malaria. Parasite Immunol. 1982 Mar;4(2):117–127. doi: 10.1111/j.1365-3024.1982.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., O'Neill P. Immunofluorescence method suitable for large-scale application to malaria. Bull World Health Organ. 1971;45(4):524–529. [PMC free article] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]


