Abstract
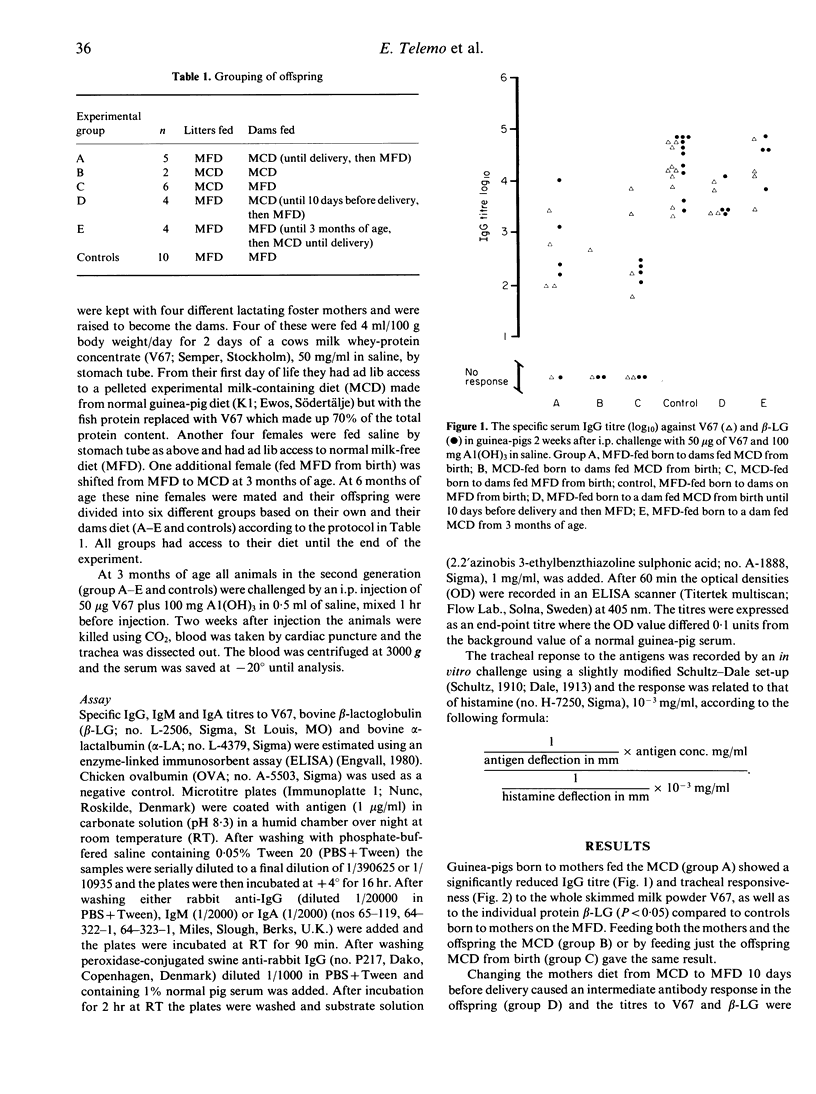
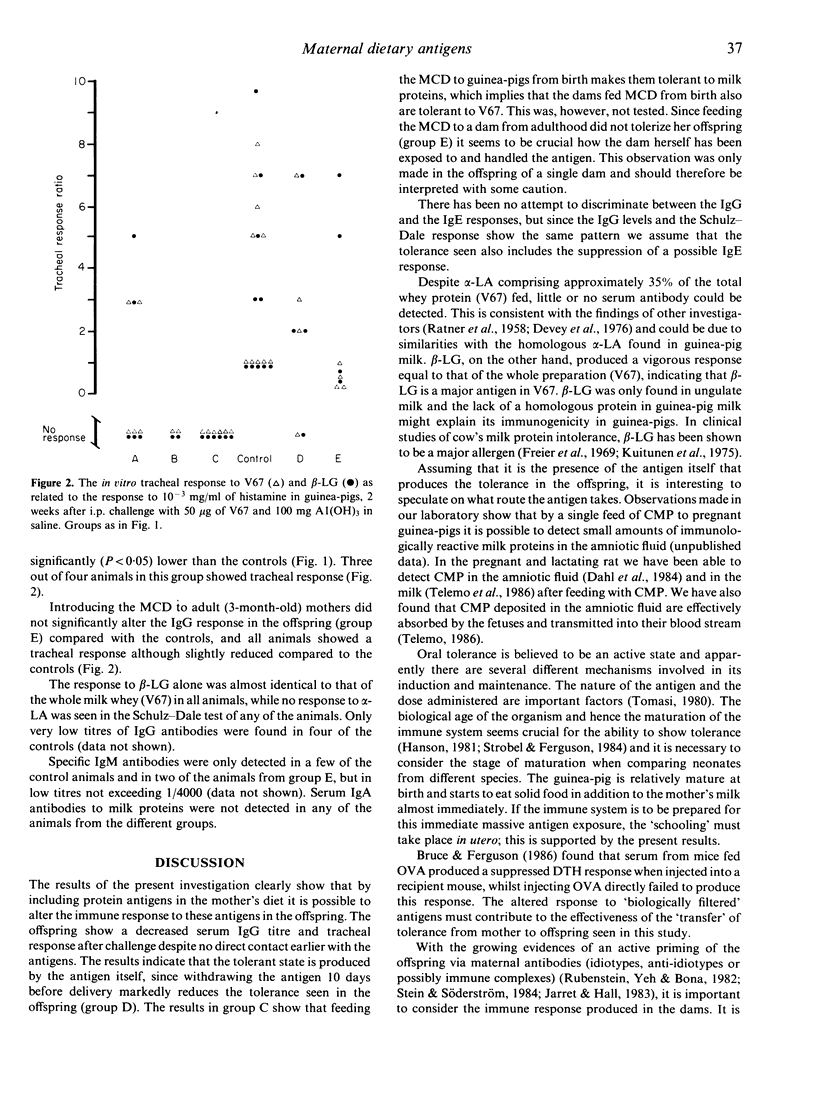
Guinea-pig dams and their litters were raised on either a cow's milk protein-containing diet (MCD) or a milk-free diet (MFD). At 8 weeks of age all litters were challenged i.p. with 50 micrograms milk whey-protein concentrate (V67) and 100 mg A1(OH)3 in saline. The immune response was estimated 2 weeks later as the serum IgG antibody titres against V67, beta-lactoglobulin (beta-LG) and alpha-lactalbumin (alpha-LA) using enzyme-linked immunosorbent assay (ELISA) and the tracheal Schulze-Dale response to these antigens. Feeding milk protein antigen to dams from birth and during pregnancy induces antigen-specific hyporesponsiveness (tolerance) in their offspring, despite no direct contact between the offspring and the milk proteins. Tolerance seems to be induced by the antigen itself since withdrawal of the MCD 10 days before delivery reduced tolerance in the offspring. No tolerance was produced in the offspring of dams fed the antigen from 3 months of age (adult). beta-LG appears to be a major antigen in milk whey while alpha-LA is a minor one since there was almost no antibody or tracheal response to alpha-LA in any of the animals tested. The results indicate that maternal antigen experience and antigens present during pregnancy are important for the subsequent immune response to these antigens in offspring.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce M. G., Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and 'biologically filtered' antigen. Immunology. 1986 Apr;57(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- Coombs R. R., Devey M. E., Anderson K. J. Refractoriness to anaphylactic shock after continuous feeding of cow's milk to guinea-pigs. Clin Exp Immunol. 1978 May;32(2):263–271. [PMC free article] [PubMed] [Google Scholar]
- Dahl G. M., Telemo E., Weström B. R., Jakobsson I., Lindberg T., Karlsson B. W. The passage of orally fed proteins from mother to foetus in the rat. Comp Biochem Physiol A Comp Physiol. 1984;77(2):199–201. doi: 10.1016/0300-9629(84)90046-x. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Anderson K. J., Coombs R. R., Henschel M. J., Coates M. E. The modified anaphylaxis hypothesis for cot death. Anaphylactic sensitization in guinea-pigs fed cow's milk. Clin Exp Immunol. 1976 Dec;26(3):542–548. [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Mowat A. M., Strobel S. Abrogation of tolerance to fed antigen and induction of cell-mediated immunity in the gut-associated lymphoreticular tissues. Ann N Y Acad Sci. 1983 Jun 30;409:486–497. doi: 10.1111/j.1749-6632.1983.tb26893.x. [DOI] [PubMed] [Google Scholar]
- Freier S., Kletter B., Gery I., Lebenthal E., Geifman M. Intolerance to milk protein. J Pediatr. 1969 Oct;75(4):623–631. doi: 10.1016/s0022-3476(69)80458-0. [DOI] [PubMed] [Google Scholar]
- Halsey J. F., Benjamin D. C. Induction of immunologic tolerance in nursing neonates by absorption of tolerogen from colostrum. J Immunol. 1976 May;116(5):1204–1207. [PubMed] [Google Scholar]
- Hanson D. G. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J Immunol. 1981 Oct;127(4):1518–1524. [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Hornbrook M. M., Lynch J. M., Roy C. A. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55(1-6):526–532. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- Heppell L. M., Kilshaw P. J. Immune responses in guinea pigs to dietary protein. I. Induction of tolerance by feeding with ovalbumin. Int Arch Allergy Appl Immunol. 1982;68(1):54–59. doi: 10.1159/000233067. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E. IgE suppression by maternal IgG. Immunology. 1983 Jan;48(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F. Effects of antigen-feeding on intestinal and systemic immune responses. II. Suppression of delayed-type hypersensitivity reactions. J Immunol. 1978 May;120(5):1509–1513. [PubMed] [Google Scholar]
- Kuitunen P., Visakorpi J. K., Savilahti E., Pelkonen P. Malabsorption syndrome with cow's milk intolerance. Clinical findings and course in 54 cases. Arch Dis Child. 1975 May;50(5):351–356. doi: 10.1136/adc.50.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Pathirana C., Goulding N. J., Gibney M. J., Pitts J. M., Gallagher P. J., Taylor T. G. Immune tolerance produced by pre- and postnatal exposure to dietary antigens. Int Arch Allergy Appl Immunol. 1981;66(1):114–118. doi: 10.1159/000232808. [DOI] [PubMed] [Google Scholar]
- Peri B. A., Rothberg R. M. Specific suppression of antibody production in young rabbit kits after maternal ingestion of bovine serum albumin. J Immunol. 1981 Dec;127(6):2520–2525. [PubMed] [Google Scholar]
- RATNER B., DWORETZKY M., OGURI S., ASCHHEIM L. Studies on the allergenicity of cow's milk. I. The allergenic properties of alpha-casein, beta-lactoglobulin and alpha-lactalbumin. Pediatrics. 1958 Sep;22(3):449–452. [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Rubinstein L. J., Yeh M., Bona C. A. Idiotype-anti-idiotype network. II. Activation of silent clones by treatment at birth with idiotypes is associated with the expansion of idiotype-specific helper T cells. J Exp Med. 1982 Aug 1;156(2):506–521. doi: 10.1084/jem.156.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C. R., Swarbrick E. T., Soothill J. F. Genetic differences in immune exclusion and partial tolerance to ingested antigens. Clin Exp Immunol. 1983 Jun;52(3):678–684. [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984 Jul;18(7):588–594. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Swarbrick E. T., Stokes C. R., Soothill J. F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut. 1979 Feb;20(2):121–125. doi: 10.1136/gut.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telemo E., Weström B., Dahl G., Karlsson B. Transfer of orally or intravenously administered proteins to the milk of the lactating rat. J Pediatr Gastroenterol Nutr. 1986 Mar-Apr;5(2):305–309. [PubMed] [Google Scholar]
- Thomas H. C., Parrott M. V. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974 Oct;27(4):631–639. [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Waters C. A., Pilarski L. M., Wegmann T. G., Diener E. Tolerance induction during ontogeny. I. Presence of active suppression in mice rendered tolerant to human gamma-globulin in utero correlates with the breakdown of the tolerant state. J Exp Med. 1979 May 1;149(5):1134–1151. doi: 10.1084/jem.149.5.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]


