Abstract
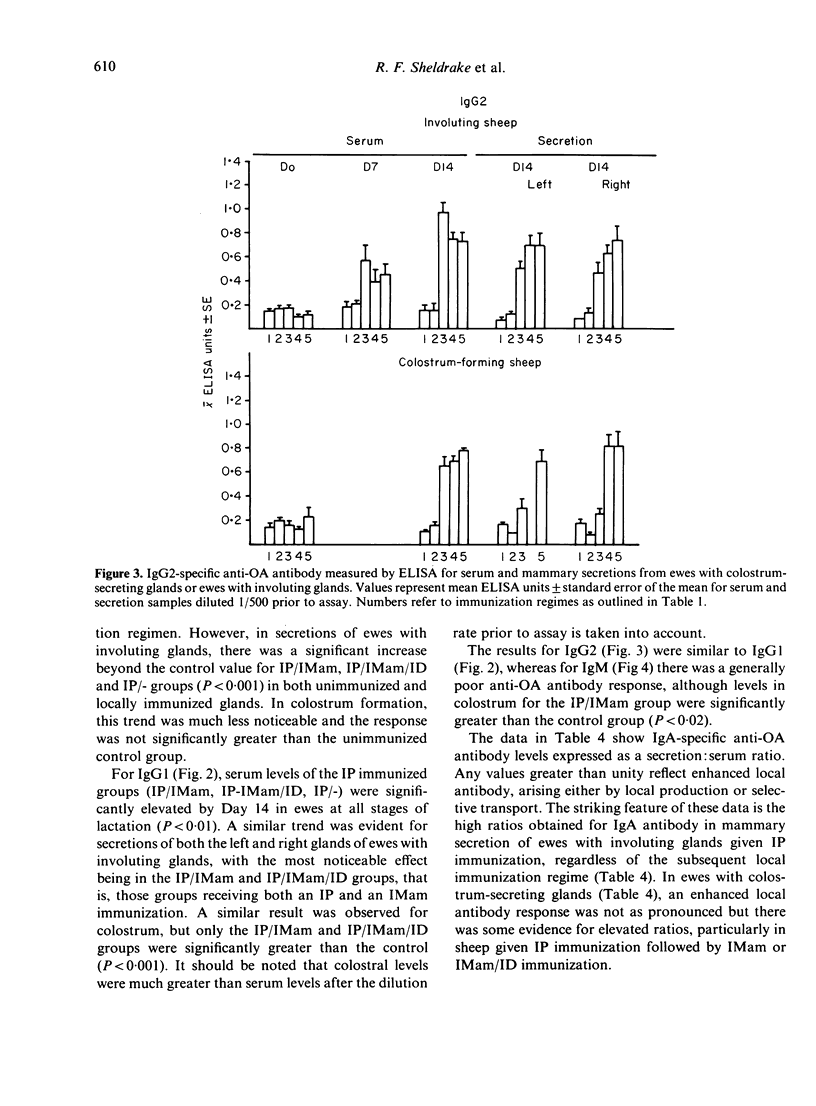
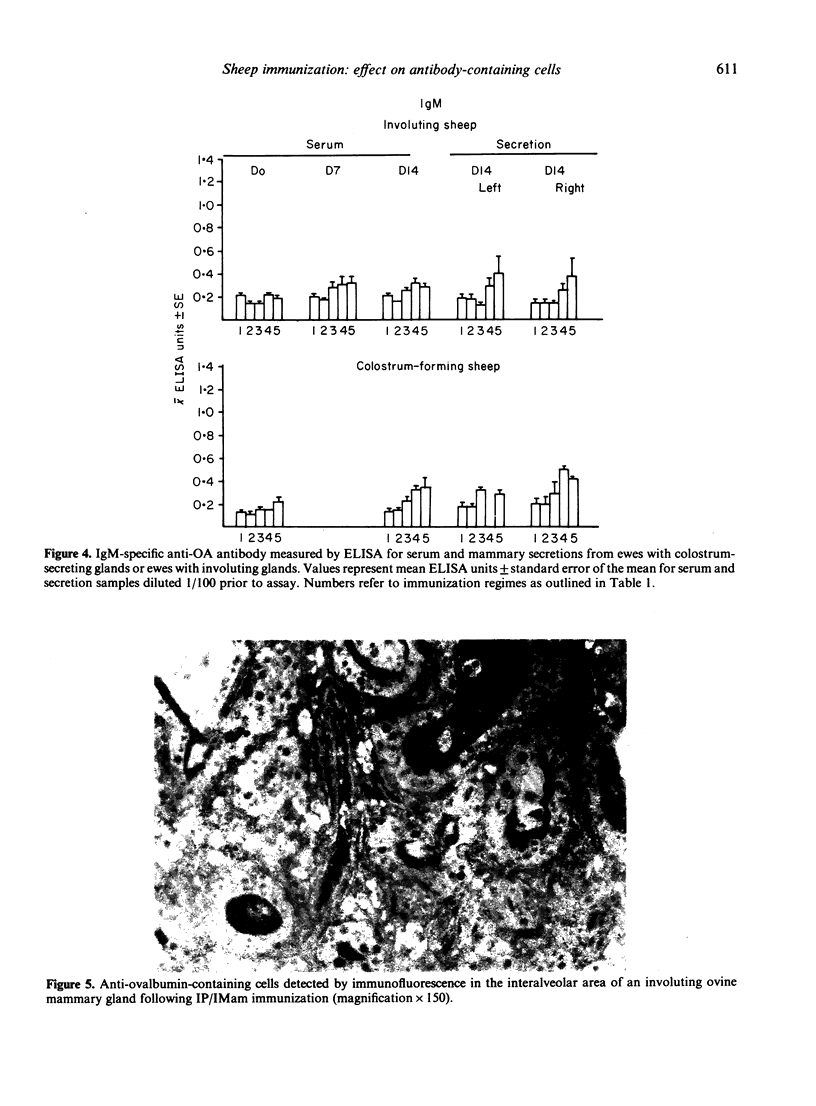
The contribution of gut-associated lymphoid tissue (GALT) to the local response in the mammary gland is well documented in laboratory animals and has been evaluated in this study in ruminants. Ewes were immunized intraperitoneally (IP) with antigen in Freund's complete adjuvant (FCA), a procedure which stimulates the production of antibodies of the IgA class in the intestine, and challenged intramammarily (IMam) either during colostrum formation or mammary gland involution. Despite a substantial IgA antibody-containing cell (ACC) response in the intestine in IP immunized sheep, there was no evidence to suggest a relocation of IgA-specific ACC to the mammary gland. There was, however, an IgA antibody response in mammary secretion of IP immunized animals, regardless of whether the mammary gland was locally immunized, but the origin of this antibody is unclear. IP/IMam immunized sheep did have an enhanced antigen-specific ACC response of the IgG1 isotype in locally immunized glands, but whether these cells were of GALT or systemic origin is also unclear.
Full text
PDF

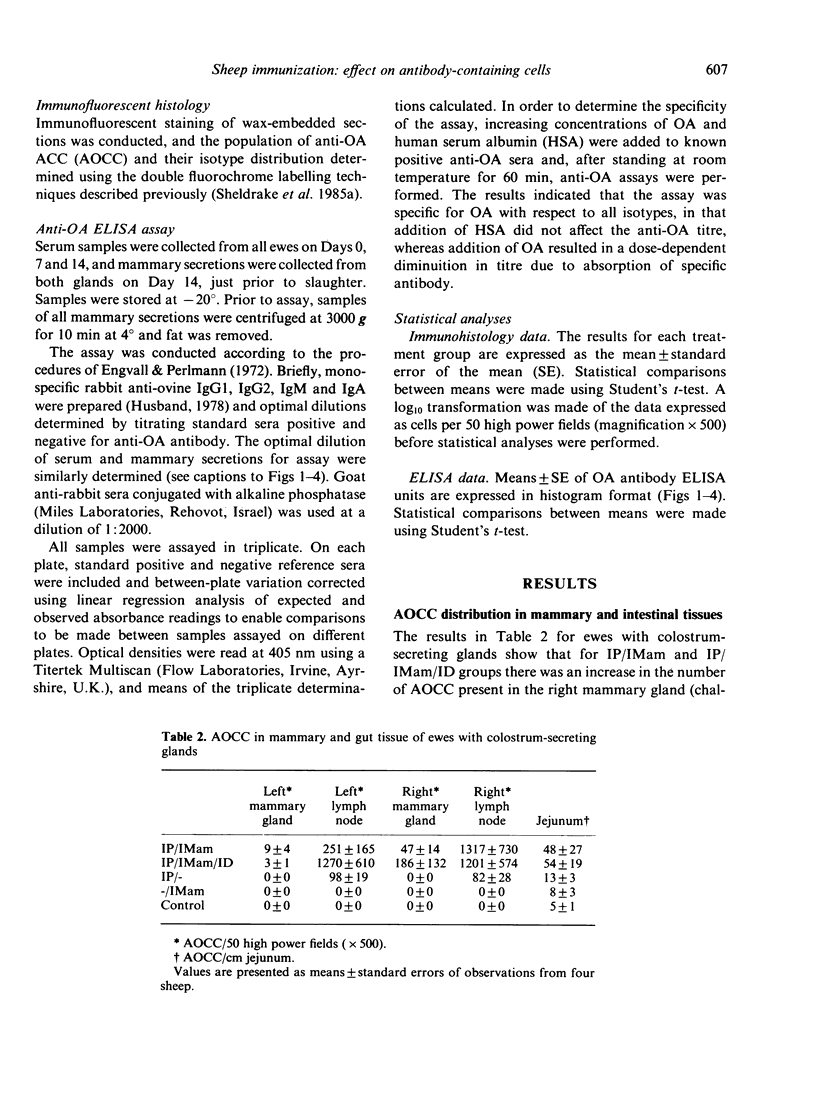
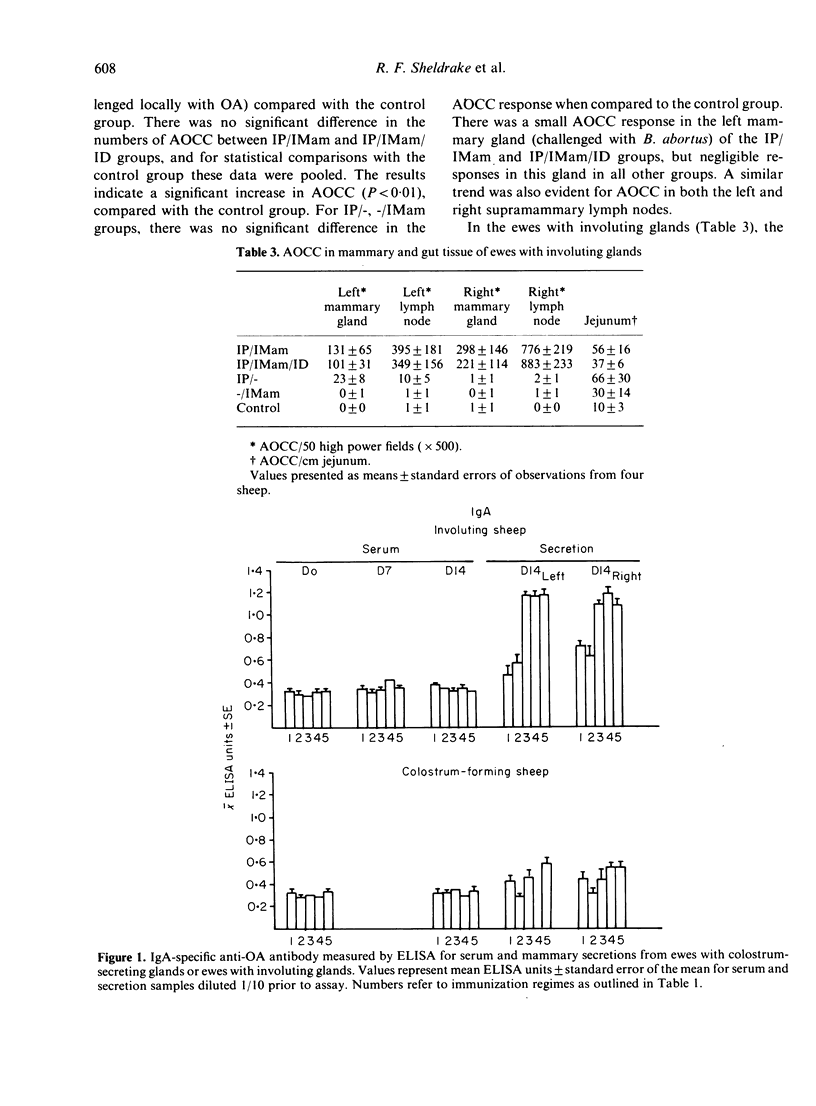
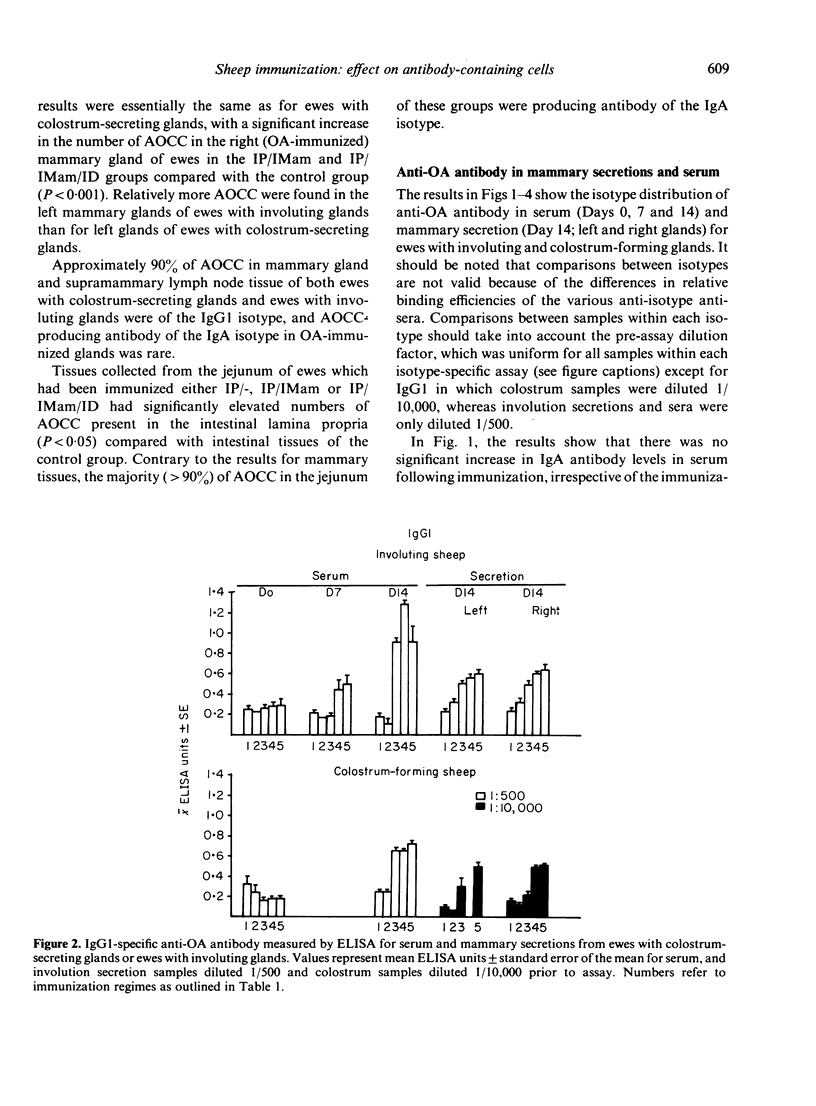



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J., Husband A. J., Lascelles A. K. Intestinal response of sheep to intraperitoneal immunization. Immunology. 1979 Jun;37(2):385–388. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J., Watson D. L., Lascelles A. K. Concentrations of immunoglobulins and albumin in lymph collected from various regions of the body of the sheep. Aust J Exp Biol Med Sci. 1974 Feb;52(1):81–86. doi: 10.1038/icb.1974.6. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Winter A. J., Norcross N. L. Immune response in the bovine mammary gland after intestinal, local, and systemic immunization. Infect Immun. 1981 Feb;31(2):650–659. doi: 10.1128/iai.31.2.650-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Husband A. J. An immunisation model for the control of infectious enteritis. Res Vet Sci. 1978 Sep;25(2):173–177. [PubMed] [Google Scholar]
- Husband A. J., Beh K. J., Lascelles A. K. IgA-containing cells in the ruminant intestine following intraperitoneal and local immunization. Immunology. 1979 Jul;37(3):597–601. [PMC free article] [PubMed] [Google Scholar]
- Jackson D. E., Lally E. T., Nakamura M. C., Montgomery P. C. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981 Sep 1;63(1):203–209. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Lascelles A. K., Beh K. J., Husband A. J. Origin of antibody in mammary secretion with particular reference to the IgA system. Adv Exp Med Biol. 1981;137:493–511. [PubMed] [Google Scholar]
- Lascelles A. K., McDowell G. H. Localized humoral immunity with particular reference to ruminants. Transplant Rev. 1974;19(0):170–208. doi: 10.1111/j.1600-065x.1974.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Lascelles A. K. Antibody-producing cells in antigenically stimulated mammary glands and in the gastro-intestinal tract of sheep. Aust J Exp Biol Med Sci. 1970 Oct;48(5):525–535. doi: 10.1038/icb.1970.52. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Scicchitano R., Husband A. J., Clancy R. L. Contribution of intraperitoneal immunization to the local immune response in the respiratory tract of sheep. Immunology. 1984 Oct;53(2):375–384. [PMC free article] [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L., Cripps A. W. Selective transport of serum-derived IgA into mucosal secretions. J Immunol. 1984 Jan;132(1):363–368. [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L. Specific antibody-containing cells in the mammary gland of non-lactating sheep after intraperitoneal and intramammary immunisation. Res Vet Sci. 1985 May;38(3):312–316. [PubMed] [Google Scholar]
- Sheldrake R. F., Scicchitano R., Husband A. J. The effect of lactation on the transport of serum-derived IgA into bile of sheep. Immunology. 1985 Mar;54(3):471–477. [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]