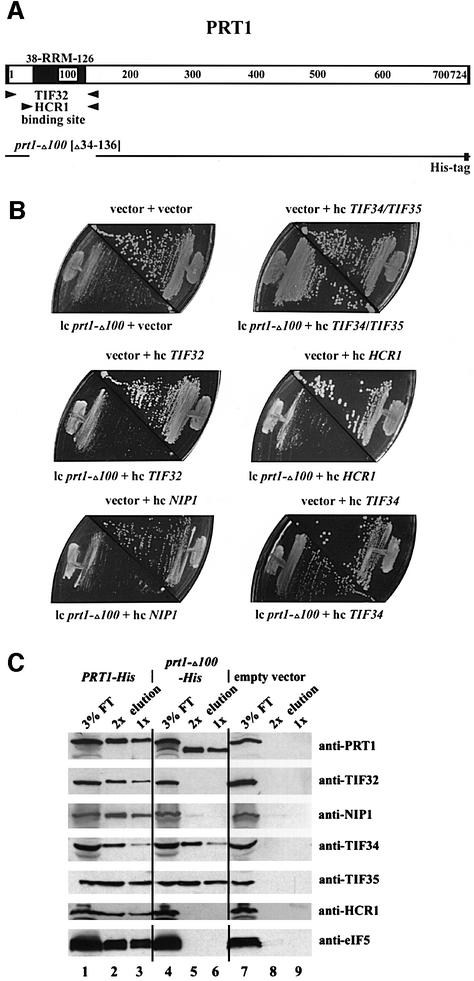
Fig. 5. The RRM of PRT1 is required for its interaction with TIF32/RPG1, HCR1 and NIP1 in vivo. (A) Schematic of the PRT1 sequence showing the locations of the RRM and minimal TIF32- and HCR1-binding sites, as in Figure 3. The line beneath the schematic depicts the amino acids present in the polyhistidine-tagged prt1-Δ100 product (prt1-Δ100-His), which lacks residues 34–136 and contains a His tag at the C-terminus. (B) The dominant Slg– phenotype of prt1-Δ100 can be suppressed by simultaneous overexpression of TIF34 and TIF35. Transformants of strain GDE303-88 containing prt1-Δ100 on low-copy-number (lc) plasmid pprt1-Δ100, or the corresponding empty vector YCplac11, were transformed additionally with one of the following high-copy-number (hc) plasmids bearing the indicated genes or with the corresponding empty vector YEp112, YEpTIF34/35T (hc TIF34/TIF35), YEpTIF32T (hc TIF32), YEpLVHCR1-1 (hc HCR1), YEpNIP1T (hc NIP1) and YEpTIF34T (hc TIF34). The resulting transformants were tested for growth at 30°C on SD medium supplemented with adenine. The plasmid combination present in each transformant is indicated above or below the appropriate plate sector. (C) Affinity purification of an eIF3 subcomplex containing prt1-Δ100-His, TIF34 and TIF35 but lacking TIF32 and NIP1. WCE was prepared in a low salt buffer (100 mM KCl) from transformants of yeast strain F353 bearing plasmid pLP101 containing PRT1-His encoding His-tagged PRT1 (lanes 1–3), pprt1-Δ100-His encoding prt1-Δ100-His (lanes 4–6) or empty vector YCplac11 (lanes 5–7). Extracts were incubated overnight with Ni2+-NTA–silica resin and the bound proteins were eluted and subjected to SDS–PAGE. Proteins were transferred to nitrocellulose membranes and probed with the antibodies indicated on the right. Lanes 1, 4 and 7 each contained 3% of the flow-through fractions from Ni2+-NTA–silica binding (3% FT); lanes 2, 3, 5, 6, 8 and 9 contained 5 µg (2×) or 2.5 µg (1×) of the corresponding eluted fractions, as indicated above the lanes.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.