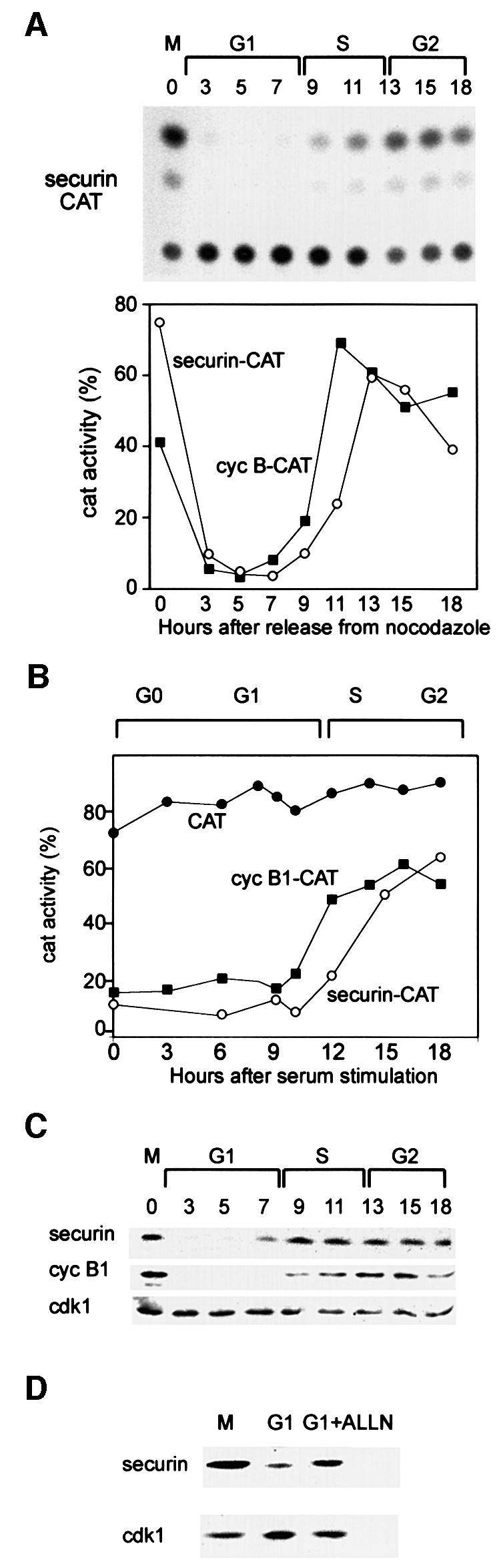
Fig. 3. Securin and cyclin B1 have a similar degradation pattern in G1. (A) Cells were stably transfected with the securin–CAT expression vector which constitutively transcribes a reporter fusion protein between the N-terminal 87 residues of human securin and a bacterial CAT protein. Cells were synchronized as described and assayed for CAT activity. The CAT activity was calculated as the ratio of the amount of diacetylated [14C]chloramphenicol to that of total [14C]chloramphenicol (diacetylated + non-acetylated) as quantified by a phosphoimager. The changes in the activities of securin–CAT (open circles) and cyclin B1–CAT (filled squares) (shown in Figure 1) were plotted as a function of the cell cycle phases when released from a nocodazole block. (B) Cells stably expressing CAT (filled circles), cyclin B1–CAT (filled squares) and securin–CAT (open circles) were arrested by serum deprivation in G0 and released by serum stimulation. Cells were harvested and analyzed for CAT activity at the indicated time points. (C) Cells were synchronized at prometaphase by nocodazole arrest and mitotic shake-off, released into fresh medium and harvested at the indicated time points for immunoblotting with securin, cyclin B1 and cdk1 antibodies. (D) Prometaphase-arrested cells were washed and released into fresh medium for 2 h, and then either harvested or grown for an additional 2 h with or without ALLN. Cell extracts were immunoblotted with securin or cdk1 antibodies. Cdk1 is constant in G1 and served as a loading control.
