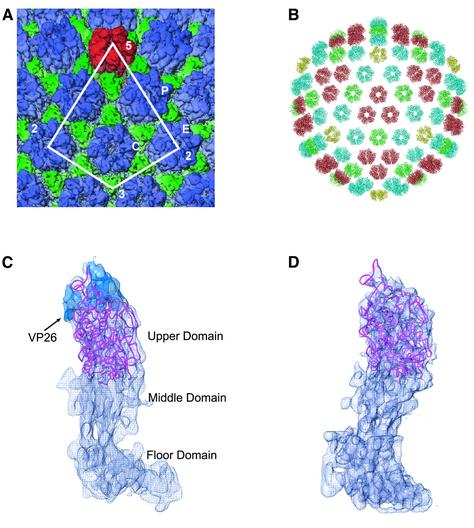
Fig. 2. Computational fitting of the VP5ud into the hexon and penton subunits. (A) Part of the 8.5 Å electron cryomicroscopy reconstruction of the HSV-1 B capsid. The penton capsomere is displayed in red, the hexon in blue and the triplex in green. The asymmetric unit of the HSV-1 nucleocapsid is enclosed by the white line. P, C and E represent the three types of hexons, the peripentonal, center and edge, respectively. (B) The crystal structure of VP5ud fitted into each of the positions it would be expected to occupy in the electron cryomicroscopy reconstruction of the HSV-1 capsid. The Cα backbones of the VP5uds are color coded yellow, red, green and blue depending on which capsomere (penton, central hexon, edge hexon or peripentonal hexon, respectively) they comprise. (C) Side view of the Cα trace of the VP5ud (magenta) modeled into the 8.5 Å electron cryomicroscopy density of a single hexon subunit. The blue density at the apical end of the VP5ud represents VP26. VP26 was delineated by calculating a difference map between the fitted VP5ud and a computationally isolated VP5 monomer from a hexon. (D) Side view of the Cα trace of the VP5ud (magenta) modeled into the 8.5 Å electron cryomicroscopy density of a single penton subunit, viewed from the same direction as in (A).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.