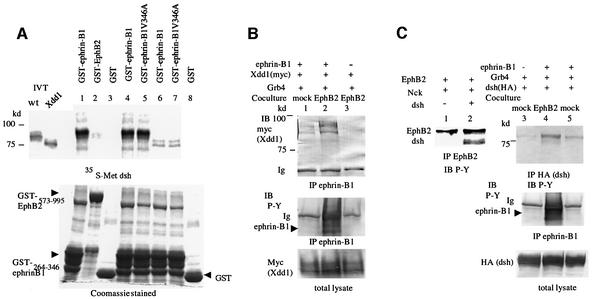
Fig. 3. (A) [35S]methionine-labeled full-length (wt, lanes 1, 2, 3, 4 and 5) or Xdd1 mutant Xdsh (lanes 6, 7 and 8) were incubated with glutathione– agarose-conjugated GST (lanes 3 and 8), GST–ephrin-B1264–346 (lanes 1, 4 and 6), GST–ephrin-B1264–346 V346A (lanes 5 and 7) or GST–EphB2573–995 (lane 2). After washing the beads, bound proteins were separated by SDS–PAGE and detected by autoradiography. IVT, input in vitro translation reaction before bead binding. Expression of GST fusion proteins is shown in the Coomassie Blue-stained gel at the bottom. (B) 293T cells stably expressing ephrin-B1 (lanes 1 and 2) or parent 293T cells (lane 3) were transiently transfected with myc-tagged Xdd1 together with Grb4. Transfected cells were co-cultured with mock-transfected 293T cells (lane 1) or 293T cells stably expressing EphB2 K661M (lanes 2 and 3) for 30 min before preparing cell lysates for immunoprecipitation with anti-ephrin-B1. Co-precipitated Xdd1 was immunoblotted with anti-myc. The phosphorylation of ephrin-B1 was shown as P-Y by anti-phosphotyrosine antibody (4G10). Ig, immunoglobulin. (C) 293T cells were transiently transfected with EphB2 and Nck with (lanes 1 and 2) or without Xdsh (lanes 3–5). 293T cells stably expressing ephrin-B1 (lanes 4 and 5) or parent 293T cells (lane 3) were transiently transfected with HA-tagged Xdsh and Grb4. Transfected cells were co-cultured with mock-tranfected 293T cells (lanes 3 and 5) or 293T cells stably expressing EphB2 K661M (lane 4) as in (B). Cell lysates were immunoprecipitated with anti-EphB2 (lanes 1 and 2) or anti-HA (lanes 3–5), and precipitated proteins were immunoblotted with anti-phosphotyrosine (P-Y: 4G10).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.