Abstract
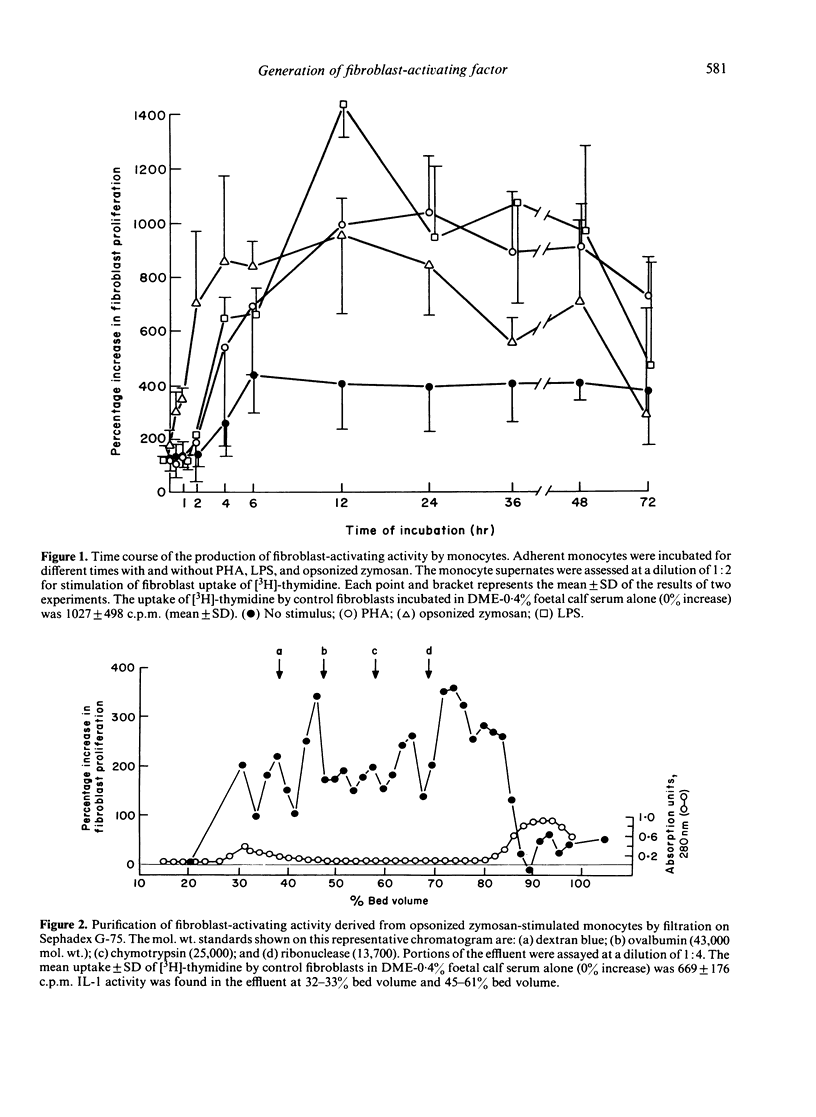
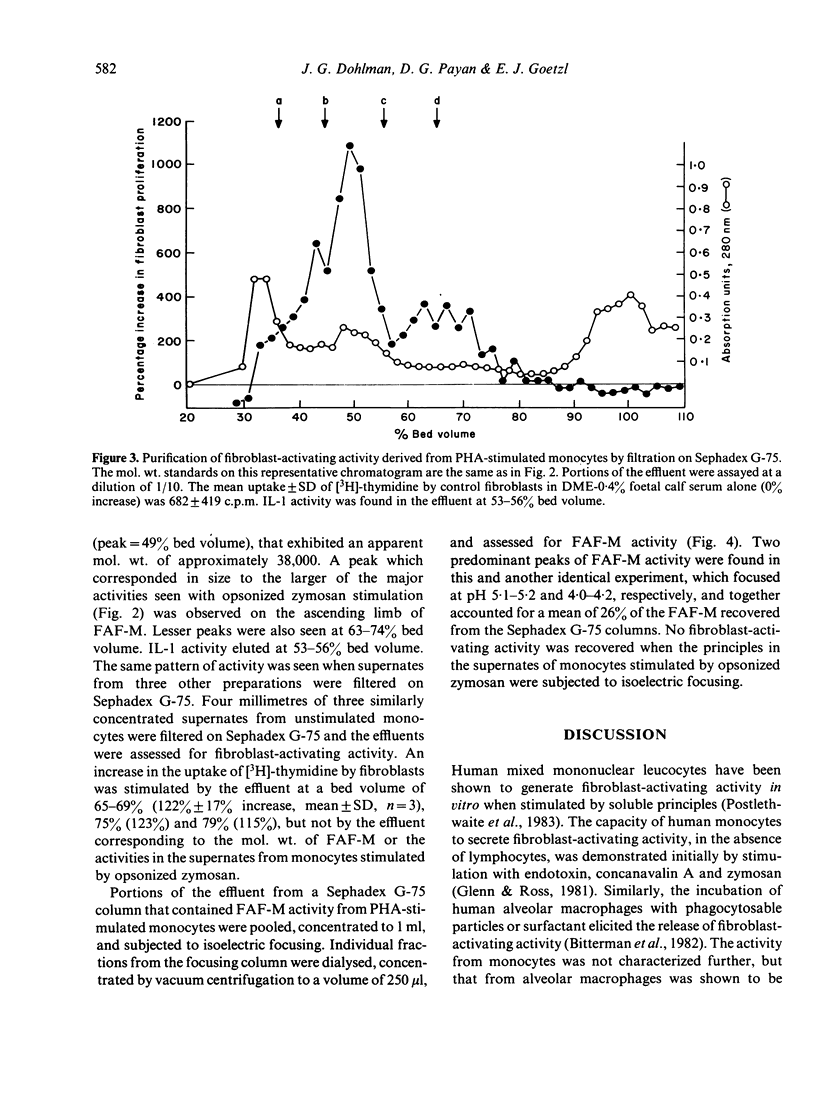
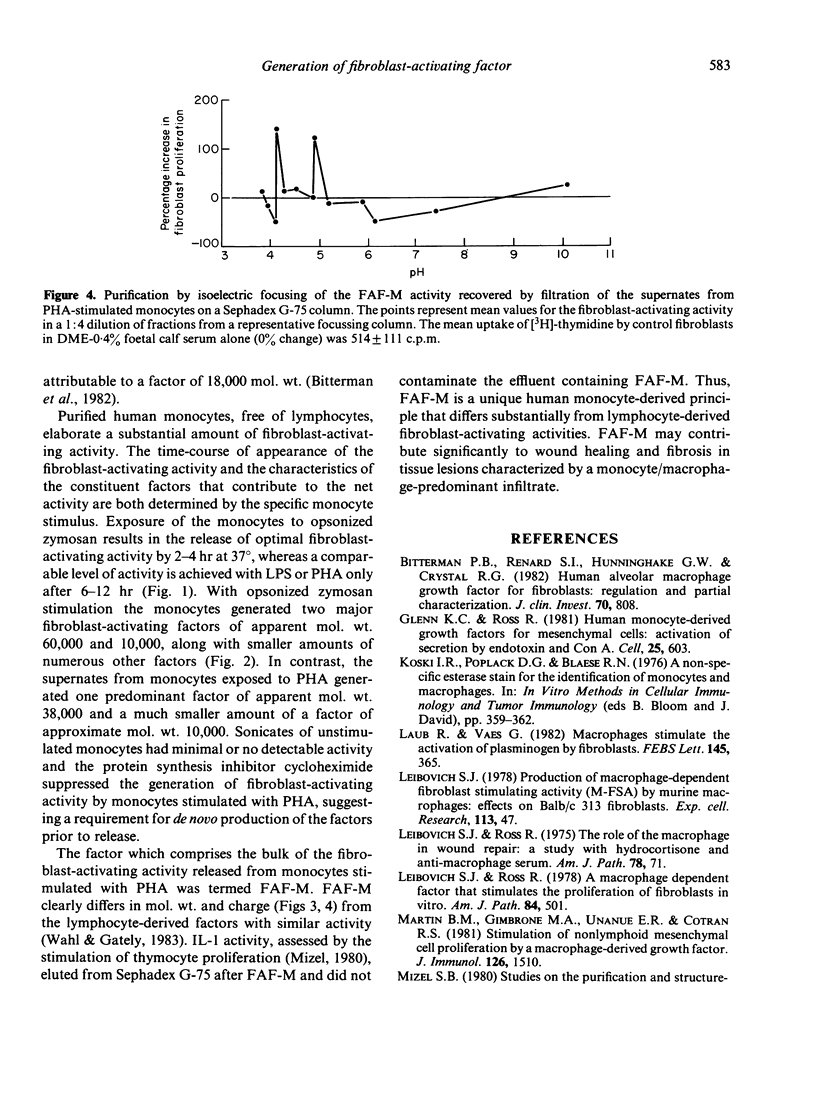
Purified human monocytes incubated with phytohemagglutinin (PHA), bacterial lipopolysaccharide (LPS), or serum-opsonized zymosan particles (OZ) generate human dermal fibroblast-activating activity, as assessed by increased fibroblast incorporation of [3H]-thymidine. A maximum concentration of fibroblast-activating activity was attained within 4 hr with OZ, whereas similar maximum levels required 12 hr with LPS and PHA. Sonicates of unstimulated monocytes had only minimal activity and the protein synthesis inhibitor cycloheximide suppressed significantly the appearance of fibroblast-activating activity, suggesting that the factors are generated prior to release. Filtration of supernates from OZ-stimulated monocytes on Sephadex G-75 yielded polydisperse fibroblast-activating activities, of which the major factors exhibited a mol. wt. of approximately 60,000 and 10,000. The supernates from PHA-stimulated monocytes had one predominant factor, termed fibroblast-activating factor of monocytes (FAF-M), with an apparent mol. wt. of 38,000 and a minor activity with a mol. wt. of 10,000. FAF-M was composed of two principles with isoelectric points of 5.1-5.2 and 4.0-4.2 and was free of interleukin-1, as determined by the absence of thymocyte-activating activity. FAF-M and other fibroblast-activating factors may contribute to wound healing and fibrosis in lesions characterized by mononuclear phagocyte infiltrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glenn K. C., Ross R. Human monocyte-derived growth factor(s) for mesenchymal cells: activation of secretion by endotoxin and concanavalin A. Cell. 1981 Sep;25(3):603–615. doi: 10.1016/0092-8674(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Laub R., Vaes G. Macrophages stimulate the activation of plasminogen by fibroblasts. FEBS Lett. 1982 Aug 23;145(2):365–368. doi: 10.1016/0014-5793(82)80201-9. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Mizel S. B. Studies on the purification and structure-functional relationships of murine lymphocyte activating factor (Interleukin 1). Mol Immunol. 1980 May;17(5):571–577. doi: 10.1016/0161-5890(80)90155-8. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Induction of fibroblast proliferation by human mononuclear leukocyte-derived proteins. Arthritis Rheum. 1983 Jan;26(1):22–27. doi: 10.1002/art.1780260104. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Tsukamoto Y., Helsel W. E., Wahl S. M. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981 Aug;127(2):673–678. [PubMed] [Google Scholar]
- Wahl S. M., Gately C. L. Modulation of fibroblast growth by a lymphokine of human T cell continuous T cell line origin. J Immunol. 1983 Mar;130(3):1226–1230. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]


