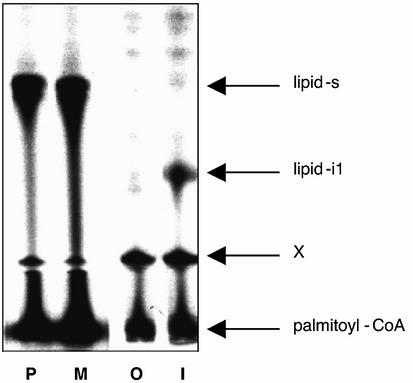
Fig. 4. Autopalmitoylation of peptides at Cys. Synthetic peptides representing the N-terminus of Gαs (P, M, O: GCLGNSK) or Gαi (I: GCTLSAEDK) were chemically modified at the N-terminal glycine by palmitate (P), myristate (M and I) or nothing (O). Peptides were subjected to autopalmitoylation and separated by thin-layer chromatography. Radioactivity was detected by fluorography. Shown is the fluorogram of a representative developed TLC plate. Arrows indicate the position of the bi-acylated N-terminal peptide of Gαs (lipid-s), bi-acylated N-terminal peptide of Gαi (lipid-i1), and the substrate palmitoyl–coenzyme A near to the origin. X denotes a non-identified spot that is already present in the substrate palmitoyl–coenzyme A and is located just above the preadsorbent/silica gel interface of the plate.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.