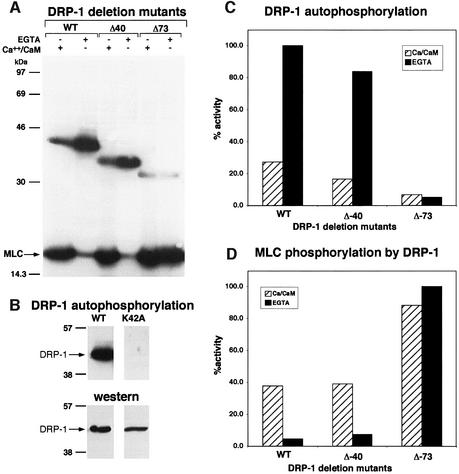
Fig. 1. The in vitro kinase activity of DRP-1 is inversely correlated with its autophosphorylation. (A) DRP-1, DRP-1 Δ40 and DRP-1 Δ73 mutant proteins were assayed in vitro for kinase activity in the presence of Ca2+/CaM or EGTA as described in Materials and methods. Autophosphorylated DRP-1 proteins (between the 30 and 46 kDa markers) and exogenous MLC substrate are shown. (B) The autophosphorylation of wild-type DRP-1 protein is compared with the catalytically inactive K42A mutant (upper part); western blot analysis with anti-FLAG antibodies showing comparable wild-type and mutant protein levels within the immunoprecipitates (lower part). (C and D) Bars showing the relative amount of autophosphorylation and MLC phosphorylation by DRP-1 deletion mutants in the presence or absence of Ca2+/CaM, after normalizing to protein expression levels. The latter was determined by incubating the same membranes with anti-HA antibodies followed by ECL detection.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.