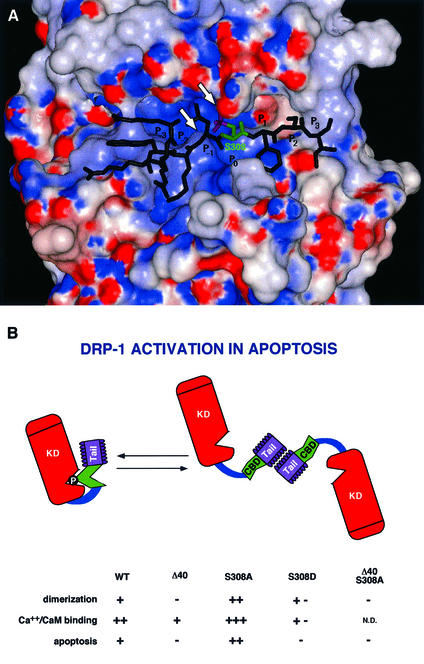
Fig. 10. (A) Three-dimensional model structure of the kinase domain of DRP-1 with a bound peptide derived from the CaM regulatory segment. The water-accessible surface of the kinase domain is coloured according to the electrostatic potential: red for negative and blue for positive potential. The peptide is shown as a stick diagram with Ser308 emphasized in green and the phosphate moiety in magenta. The arrowheads point to the ATP-binding P-loop and to Lys141. (B) A scheme of DRP-1 structural motifs. The scheme provides a summary of the different features that change by the point mutations or the deletions including the dimerization, CaM-binding and apoptotic functions. The various domains are marked by KD (kinase domain), CBD (CaM-binding domain) and Tail (the C-terminal 40-amino-acid peptide). The catalytic cleft is marked by a V-shaped structure in which the phosphate residue on Ser308 resides. N.D., not done.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.