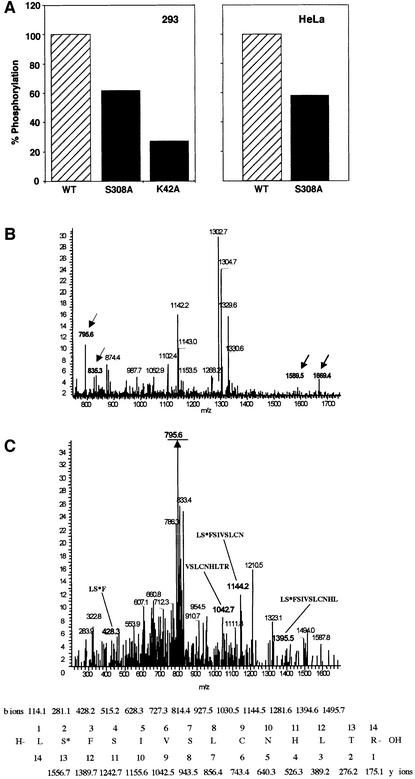
Fig. 8. Ser308 is auto-phosphorylated in vivo. (A) 293 cells, transfected with wild type, S308A and K42A DRP-1 mutants (left), and HeLa cells, transfected with wild type and S308A mutants (right), were subjected to in vivo labelling with [33P]orthophosphate as described in Materials and methods. Proteins were extracted and the levels of 33P incorporated into DRP-1 were quantitated after normalization to the protein expression levels. (B and C) Mass spectrometric analysis of ectopically expressed DRP-1 protein extracted from growing Hela cells. (B) DRP-1 tryptic peptides were analysed by liquid chromatography–mass spectrometry. Full mass spectrum at retention time 60 min. The masses corresponding to singly and doubly charged ions of peptide 307–320 in the unphosphorylated (m/z = 1589 and 795.6) and the phosphorylated (m/z = 1669.4 and 835.3) forms are indicated by arrows. (C) CID of the m/z = 835.3 peptide and comparison with the simulated CID of phosphorylated 307–320 DRP-1 peptide. The major fragment m/z = 795.6 (underlined) correlates with the doubly charged mass of the full peptide after HPO3 removal (indicating the existence of a single phosphate residue on this peptide). The relevant CID fragments are marked in bold and their amino acid sequence and masses are shown at the bottom. The phosphoserine is marked as S*.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.