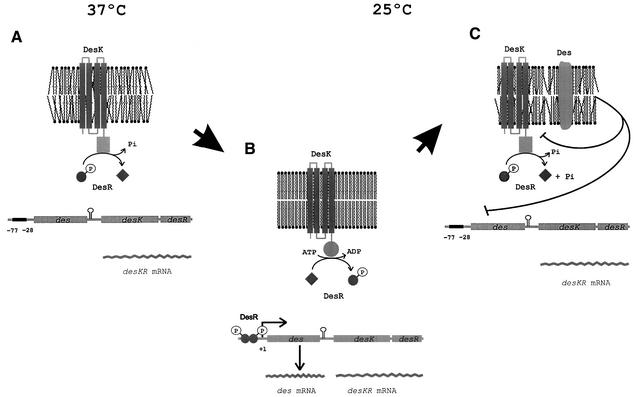
Fig. 8. Model of des transcriptional control by two-component temperature signal transduction proteins. It is proposed that DesK assumes different signalling states in response to a temperature-induced change in membrane fluidity. This is accomplished by regulating the ratio of kinase to phosphatase activity such that a phosphatase-dominant state is present at 37°C, when membrane lipids are disordered (A), whereas a kinase-dominant state predominates upon an increase in the proportion of ordered membrane lipids after a temperature downshift to 25°C (B). DesK-mediated phosphorylation of DesR results in transcriptional activation of des (B). Activation of des results in synthesis of Des, which desaturates the acyl chains of membrane phospholipids (C). These newly synthesized UFAs inhibit des transcription either by favouring DesK dephosphorylation of DesR-P or by causing dissociation of DesR-P from its binding site (C) (see text for further details).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.