Abstract
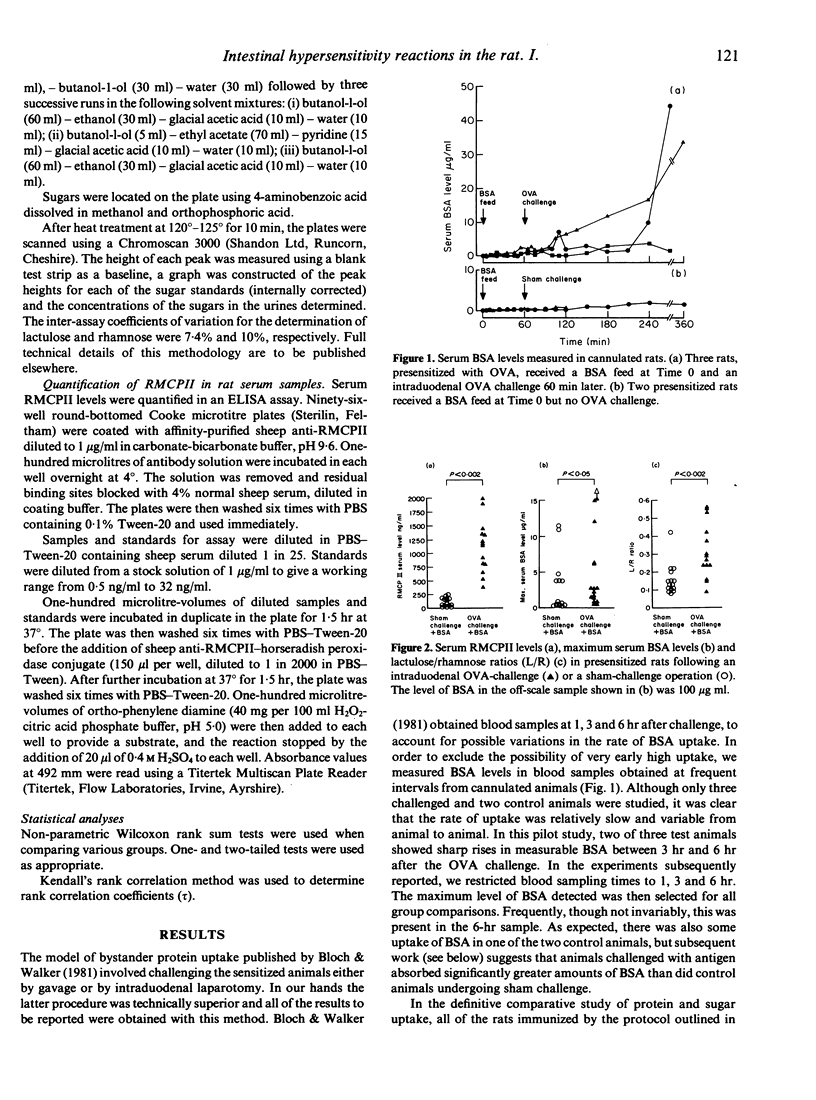
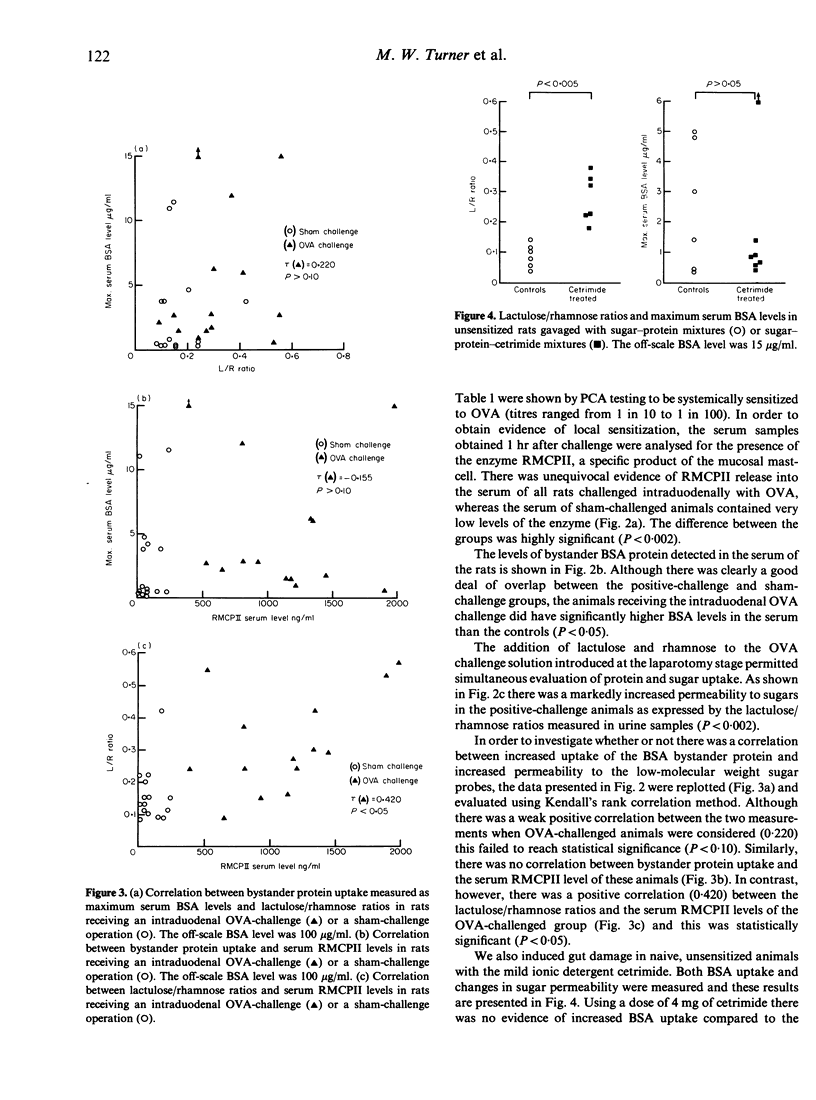
We have confirmed previous observations that intestinal anaphylaxis induced in rats previously sensitized to ovalbumin (OVA) is associated with an increased uptake of an unrelated 'bystander' protein, bovine serum albumin (BSA) fed 1 hr previously. In this study, this enhanced protein uptake was associated with an increased lactulose/rhamnose excretion ratio after administration of these sugars, although there was no correlation between the two measurements. One hour after antigen challenge the serum levels of rat mast-cell protease II (RMCPII), a specific marker for mucosal mast-cell secretion, were significantly higher than both the pre-challenge levels and those of sham-challenged controls (P less than 0.002). There was a significant positive correlation between the serum levels of RMCPII and the lactulose/rhamnose excretion ratios (P less than 0.05), but no such correlation existed between RMCPII and BSA levels in the challenged rats. In other studies the urinary lactulose/rhamnose ratios of rats with cetrimide-induced gut damage were found to be significantly increased, although BSA uptake into the serum remained unaltered. We conclude that there is no simple correlation between gut permeation of low-molecular weight sugars and and the uptake of macromolecular proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch K. J., Walker W. A. Effect of locally induced intestinal anaphylaxis on the uptake of a bystander antigen. J Allergy Clin Immunol. 1981 Apr;67(4):312–316. doi: 10.1016/0091-6749(81)90027-0. [DOI] [PubMed] [Google Scholar]
- Byars N. E., Ferraresi R. W. Intestinal anaphylaxis in the rat as a model of food allergy. Clin Exp Immunol. 1976 May;24(2):352–356. [PMC free article] [PubMed] [Google Scholar]
- Cobden I., Rothwell J., Axon A. T. Passive permeability in experimental intestinal damage in rats. Clin Sci (Lond) 1981 Jan;60(1):115–118. doi: 10.1042/cs0600115. [DOI] [PubMed] [Google Scholar]
- Dannaeus A., Inganäs M., Johansson S. G., Foucard T. Intestinal uptake of ovalbumin in malabsorption and food allergy in relation to serum IgG antibody and orally administered sodium cromoglycate. Clin Allergy. 1979 May;9(3):263–270. doi: 10.1111/j.1365-2222.1979.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Jr, Ewton M. F., Soter N., Kinney J. Permeability characteristics of the human small intestine. J Clin Invest. 1965 Dec;44(12):1935–1944. doi: 10.1172/JCI105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby S., Jensenius J. C., Svehag S. E. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985 Jul;22(1):83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Jackson P. G., Lessof M. H., Baker R. W., Ferrett J., MacDonald D. M. Intestinal permeability in patients with eczema and food allergy. Lancet. 1981 Jun 13;1(8233):1285–1286. doi: 10.1016/s0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Slade H. Passage of ingested protein into the blood during gastrointestinal hypersensitivity reactions: experiments in the preruminant calf. Clin Exp Immunol. 1980 Sep;41(3):575–582. [PMC free article] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Miller H. R., Woodbury R. G., Huntley J. F., Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983 Jul;49(3):471–479. [PMC free article] [PubMed] [Google Scholar]
- Paganelli R., Atherton D. J., Levinsky R. J. Differences between normal and milk allergic subjects in their immune responses after milk ingestion. Arch Dis Child. 1983 Mar;58(3):201–206. doi: 10.1136/adc.58.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli R., Levinsky R. J., Atherton D. J. Detection of specific antigen within circulating immune complexes: validation of the assay and its application to food antigen-antibody complexes formed in healthy and food-allergic subjects. Clin Exp Immunol. 1981 Oct;46(1):44–53. [PMC free article] [PubMed] [Google Scholar]
- Paganelli R., Levinsky R. J., Brostoff J., Wraith D. G. Immune complexes containing food proteins in normal and atopic subjects after oral challenge and effect of sodium cromoglycate on antigen absorption. Lancet. 1979 Jun 16;1(8129):1270–1272. doi: 10.1016/s0140-6736(79)92230-x. [DOI] [PubMed] [Google Scholar]
- Pike M. G., Heddle R. J., Boulton P., Turner M. W., Atherton D. J. Increased intestinal permeability in atopic eczema. J Invest Dermatol. 1986 Feb;86(2):101–104. doi: 10.1111/1523-1747.ep12284035. [DOI] [PubMed] [Google Scholar]
- Strobel S., Brydon W. G., Ferguson A. Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut. 1984 Nov;25(11):1241–1246. doi: 10.1136/gut.25.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagesson C., Franzén L., Dahl G., Weström B. Lysophosphatidylcholine increases rat ileal permeability to macromolecules. Gut. 1985 Apr;26(4):369–377. doi: 10.1136/gut.26.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]


