Figure 1.
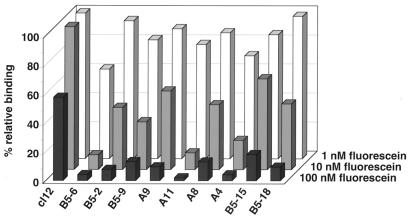
Analysis of off-rate selections: semiquantitative inhibition RIA of selected c12 mutants (for details see Experimental Protocol). Kinetic and affinity parameters of purified c12 and three mutants were determined experimentally as described in the text. Because of the similar RIA profile of all mutants three c12 mutants of this set along with the parental protein were further analyzed. c12 wild-type: koff = 0.0028 s−1, kon = 2.5 × 106 M−1 s−1, KD, calc = 1.1 nM, KD, titr = 1.5 nM; c12 B5–6: koff = 0.00014 s−1, kon = 3.4 × 106 M−1 s−1, KD, calc = 0.04 nM, KD,titr = 0.1 nM; c12 B5–2: koff = 0.00020 s−1; c12 B5–9: koff = 0.00018 s−1. Measurements were performed at 25°C as described (19). KD, calc is the ratio obtained from the dissociation rate (koff) and the association rate (kon), and KD, titr is the equilibrium dissociation constant directly determined by titration. The error between duplicate measurements for koff and kon of all measured mutants is about ±3%; the error for KD, titr is about ±8%.