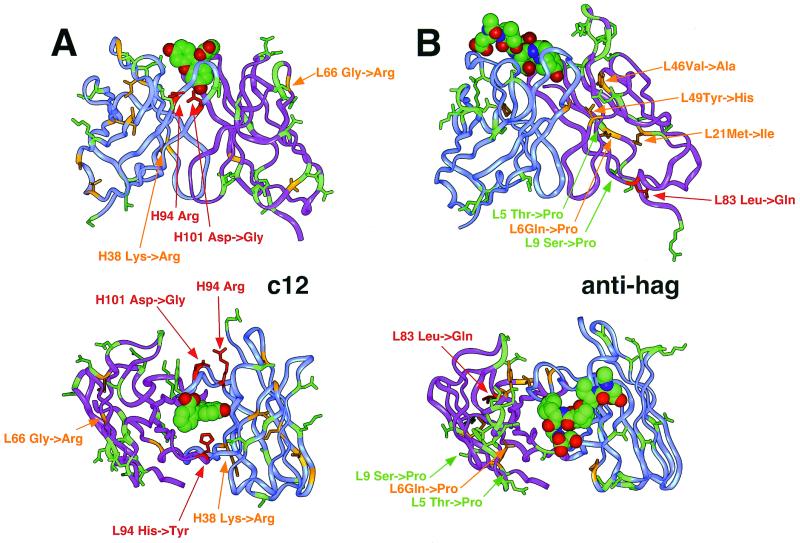
Figure 3.
Localization of the mutated residues in a three-dimensional model of c12 with docked fluorescein (theoretical model obtained by homology modeling, based on Protein Data Bank (PDB) entries 1iai, 1nca, 1igc for VL; 1for, 1plg for VH; and 1mim for CDR-H3) (A) and in the anti-influenza hemagglutinin antibody Fab 17/9 [experimental structure, PDB entry 1ifh (34)] (B). VH is shown in blue; VL is in purple. The strongly selected mutations anti-hag L83 Leu to Gln, c12 L94 His to Tyr, and c12 H101 Asp to Gly, Ser, or Ala and its salt-bridge partner Arg H94 are indicated in red. The positions of the remaining mutated residues are indicated by orange if the affected side chains are buried in the domain core and by green if they are located on the surface of the molecule.