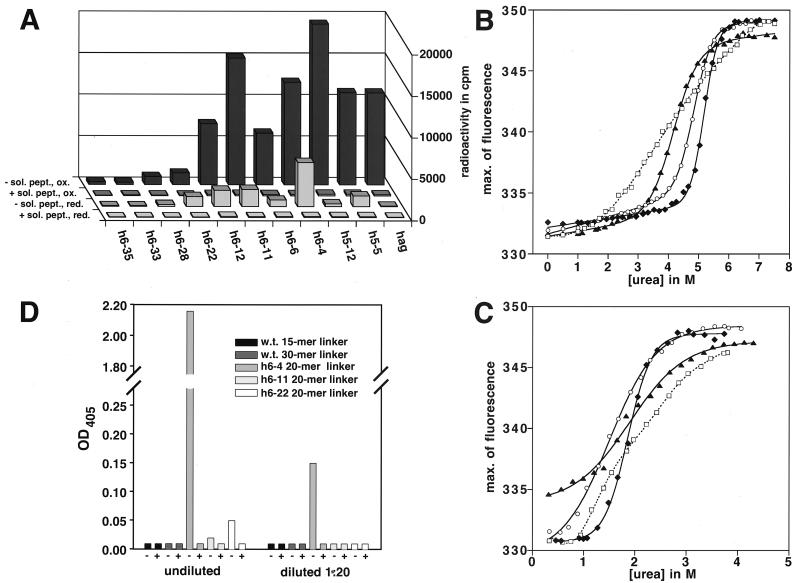
Figure 4.
Analysis of stability-matured hag mutants after in vivo and in vitro expression. Absolute RIA signal after reducing and oxidizing in vitro translation (A) and demonstration of specific binding by inhibition with 1 μM soluble hag peptide signals from in vitro translation under oxidizing conditions (ox) or reducing conditions (red) are shown. + or −sol. peptide indicates the presence or absence of 1 μM hag peptide as a competitor. By subtraction of the radioactivity of samples that were inhibited from the radioactivity of the uninhibited samples, the inhibitable portion of the RIA signal was obtained as a measure for specific binding activity. The resulting ratios of relative binding are calculated by dividing the inhibitable portion of the RIA signal in the presence of 10 mM DTT by the inhibitable RIA signal in the absence of DTT. They are as follows: anti-hag scFv wild-type: 0.2%; h5–5: 10.9%; h5–12: 1.6%; h6–4: 40.8%; h6–6: 11.1%; h6–11: 13.1%; h6–12: 26.4%; h6–22: 84.5%; h6–28: 20.9%; h6–33: 17.9%; h6–35: 17.0%. Urea denaturation at 10°C (B) of periplasmically produced hag wild-type and mutant proteins. ▴, Anti-hag wild-type scFv; ○, mutant h6–11; □, mutant h6–4; ⧫, mutant h6–22. (C) Urea equilibrium renaturation under reducing conditions at 20°C of periplasmically produced hag wild-type and mutant proteins. (D) Normalized crude cell lysate ELISA after expression of hag wild-type and mutant proteins at 25°C in the cytoplasm of E. coli. Bars represent the binding signals of active protein in the absence (−) and presence (+) of competing soluble hag-peptide (1 μM), to indicate specific binding.