Abstract
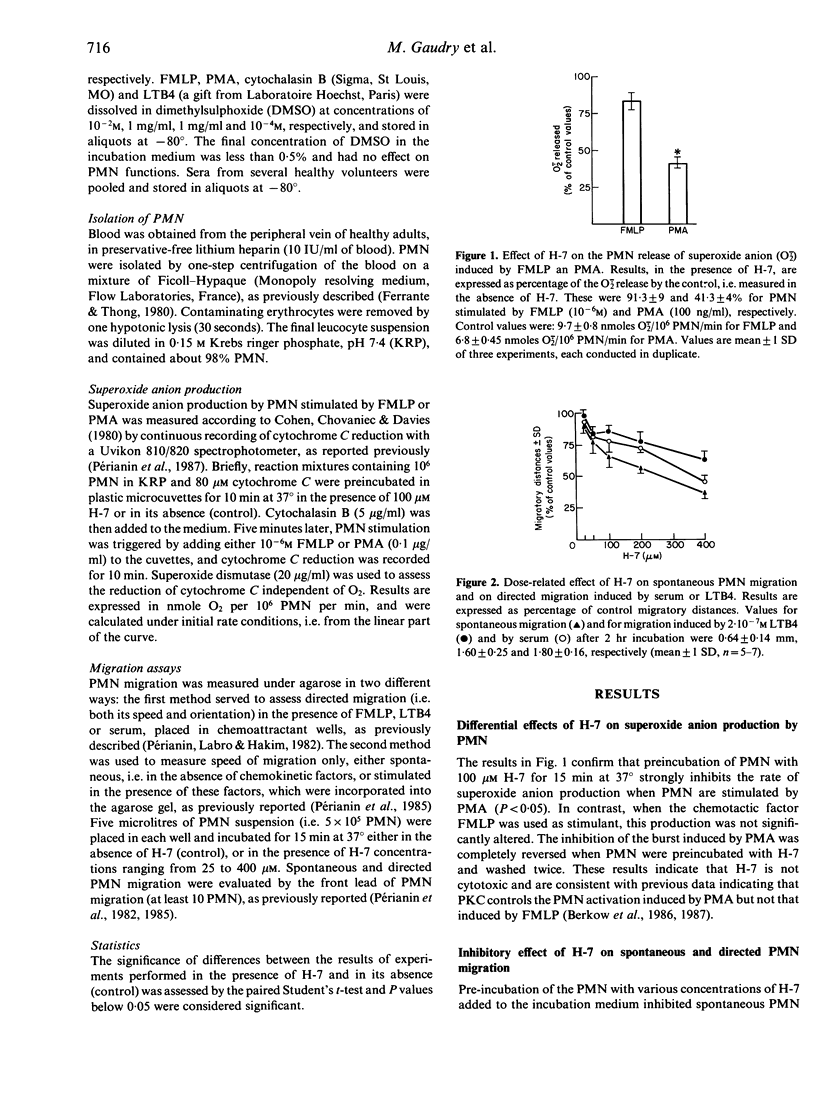
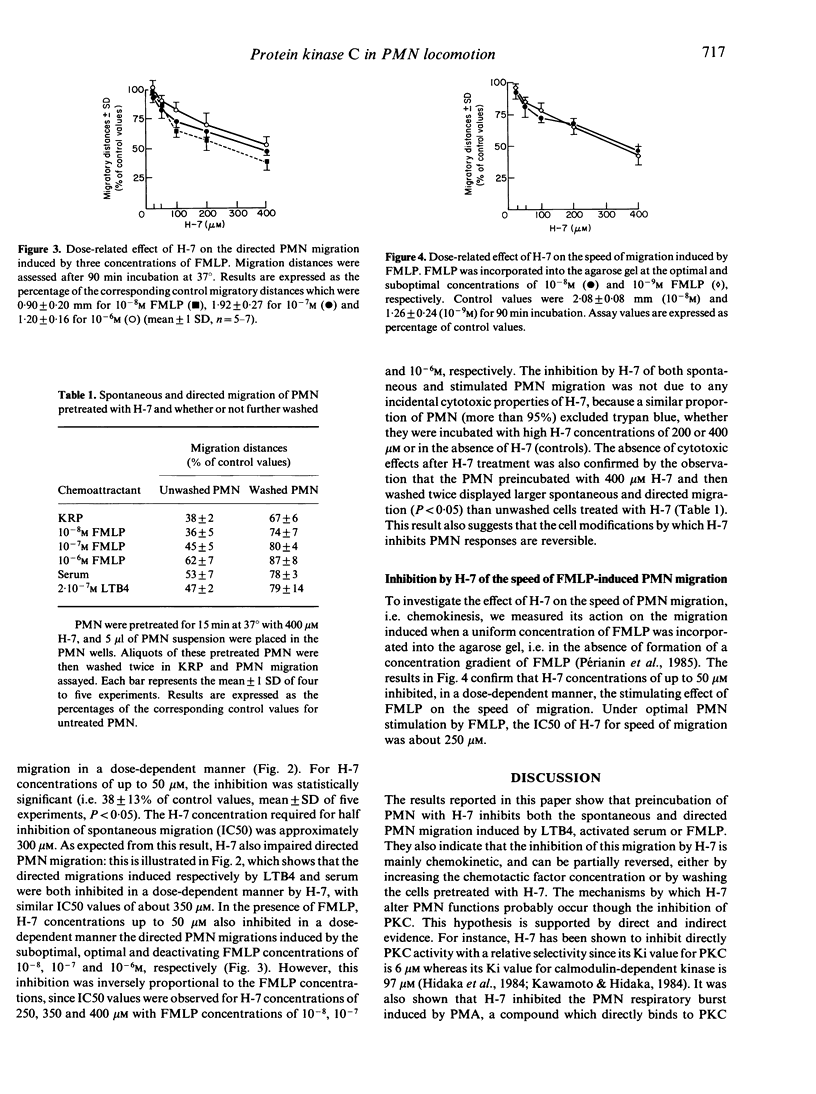
The effects of 1-(5-isoquinoline sulphonyl)-2-methyl piperazine (H-7), a recently described inhibitor of Ca2+/phospholipid-dependent protein kinase (protein kinase C), were studied during under-agarose migration of human polymorphonuclear neutrophils (PMN) stimulated by various chemoattractants, in order to determine whether protein kinase C is involved in PMN locomotion. The effect of H-7 on the oxidative burst induced by phorbol 12-myristate 13-acetate or N-formyl-methionyl-leucyl-phenylalanine (FMLP) was also measured. Pre-incubation of PMN with H-7 concentrations ranging from 50 to 400 microM inhibited: (i) spontaneous PMN migration under agarose; (ii) the directed migration induced by activated serum, leukotriene B4 or FMLP; and (iii) the speed of the migration induced by FMLP. The inhibition by H-7 of FMLP-induced directed migration was less when FMLP was used at high concentrations which, in the absence of H-7, inhibit locomotion. H-7 depressed the oxidative burst induced by phorbol myristate acetate (PMA) but not that induced by FMLP. All the effects of H-7 on the oxidative burst and migration were reversed by washing PMN after H-7 treatment. These findings indicate that protein kinase C, inhibitable by H-7, is involved in a mechanism controlling the speed of PMN locomotion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkow R. L., Dodson R. W., Kraft A. S. The effect of a protein kinase C inhibitor, H-7, on human neutrophil oxidative burst and degranulation. J Leukoc Biol. 1987 May;41(5):441–446. doi: 10.1002/jlb.41.5.441. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E., Davies W. A. Activation of the guinea pig granulocyte NAD(P)H-dependent superoxide generating enzyme: localization in a plasma membrane enriched particle and kinetics of activation. Blood. 1980 Mar;55(3):355–363. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Katoh N., Wise B. C., Kuo J. F. Phosphorylation of cardiac troponin inhibitory subunit (troponin I) and tropomyosin-binding subunit (troponin T) by cardiac phospholipid-sensitive Ca2+-dependent protein kinase. Biochem J. 1983 Jan 1;209(1):189–195. doi: 10.1042/bj2090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. Ca2+-activated, phospholipid-dependent protein kinase catalyzes the phosphorylation of actin-binding proteins. Biochem Biophys Res Commun. 1984 Feb 14;118(3):736–742. doi: 10.1016/0006-291x(84)91456-6. [DOI] [PubMed] [Google Scholar]
- Nishihira J., McPhail L. C., O'Flaherty J. T. Stimulus-dependent mobilization of protein kinase C. Biochem Biophys Res Commun. 1986 Jan 29;134(2):587–594. doi: 10.1016/s0006-291x(86)80460-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Perianin A., Gougerot-Pocidalo M. A., Giroud J. P., Hakim J. Diclofenac binding to human polymorphonuclear neutrophils: effect on respiratory burst and N-formylated peptide binding. Biochem Pharmacol. 1987 Aug 15;36(16):2609–2615. doi: 10.1016/0006-2952(87)90539-9. [DOI] [PubMed] [Google Scholar]
- Perianin A., Gougerot-Pocidalo M. A., Giroud J. P., Hakim J. Diclofenac sodium, a negative chemokinetic factor for neutrophil locomotion. Biochem Pharmacol. 1985 Oct 1;34(19):3433–3438. doi: 10.1016/0006-2952(85)90714-2. [DOI] [PubMed] [Google Scholar]
- Perianin A., Labro M. T., Hakim J. Chemokinetic activity of N-formyl-methionyl-leucyl-phenylalanine on human neutrophils, and its modulation by phenylbutazone. Biochem Pharmacol. 1982 Oct 1;31(19):3071–3076. doi: 10.1016/0006-2952(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol. 1984;2:257–281. doi: 10.1146/annurev.iy.02.040184.001353. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]


