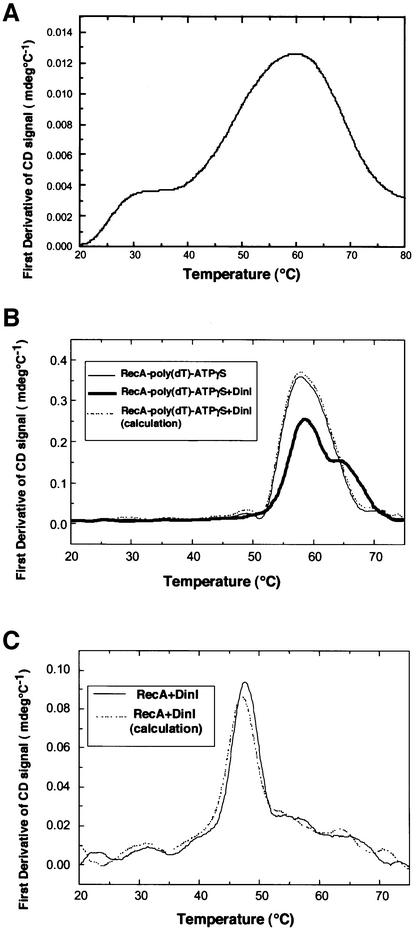
Fig. 4. Effect of DinI on the thermal stability of RecA. Thermal unfolding of DinI and RecA proteins was detected by CD change upon temperature elevation, and presented by its first derivative, d(CD)/dT. (A) DinI alone (8.3 µM). (B) RecA–poly(dT)–ATPγS complex (thin line); mixture of DinI and RecA–poly(dT)–ATPγS complex (thick line); expected theoretical curve of the mixture when there is no interaction between DinI and RecA–poly(dT)–ATPγS complex (broken line). RecA, 8.3 µM; DinI, 8.3 µM; poly(dT), 24.9 µM. (C) RecA with ATPγS (solid line) in the absence of ssDNA; expected theoretical curve of the mixture when there is no interaction between DinI and RecA with ATPγS (broken line). RecA, 4 µM; DinI, 4 µM.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.