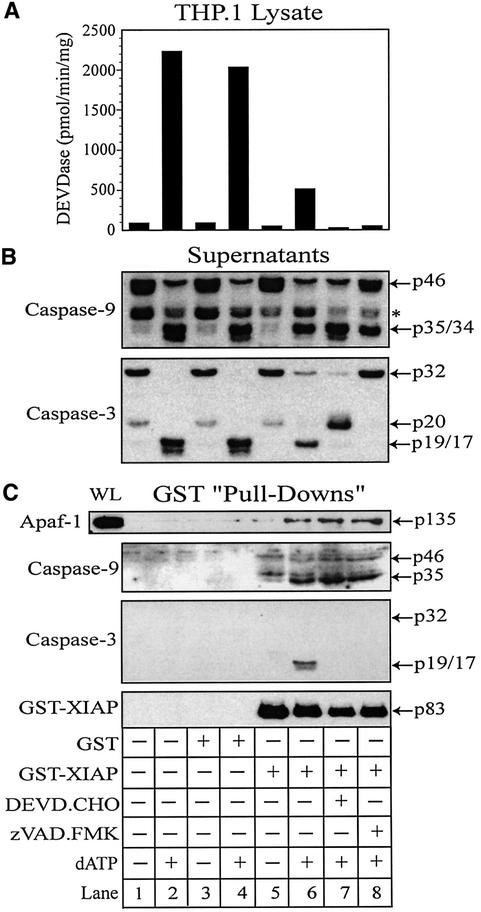
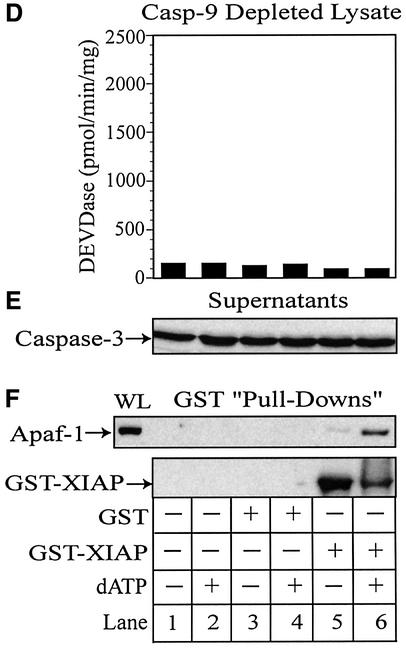
Fig. 6. XIAP directly associates with Apaf-1 and retains active caspase-3 within the apoptosome. THP.1 lysates were activated with dATP (lanes 2, 4, 6, 7 and 8) for 1 h at 37°C in the presence of GST (lanes 3 and 4) or GST–XIAP (lanes 5–8). In some treatments, the lysate was pretreated for 15 min with DEVD⋅CHO (200 nM) (lane 7) or z-VAD⋅FMK (25 µM) (lane 8). A small aliquot of each sample was immediately assayed for (A) DEVDase activity and the remainder incubated with GSH–Sepharose beads (20 µl) overnight at 4°C. Following centrifugation, supernatants were collected and the beads thoroughly washed (four times) with assay buffer. (B) Supernatants and (C) GST–XIAP/protein complexes were subsequently mixed with 2× SDS loading buffer, separated by SDS–PAGE and immunoblotted for Apaf-1, caspase-9, caspase-3 and/or XIAP. Similarly, caspase-9-depleted lysates were activated with dATP (lanes 2, 4 and 6) in the presence of GST (lanes 3 and 4) or GST–XIAP (lanes 5 and 6). A small aliquot of each sample was assayed for (D) DEVDase activity and the remainder incubated with GSH–Sepharose beads (20 µl) overnight at 4°C. After centrifugation, the resulting (E) supernatants and (F) GST–XIAP/protein complexes were separated by SDS–PAGE and immunoblotted for Apaf-1, caspase-3 and/or XIAP, as described above. All Apaf-1 western blots are presented at the same exposures; ‘WL’ represents the total amount of Apaf-1 present in each whole lysate that was available to interact with GST–XIAP.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.