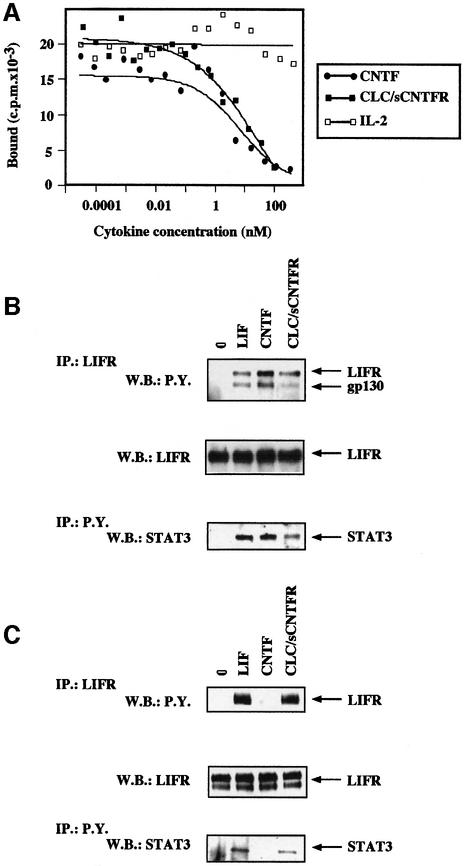
Fig. 6. Competition of CLC–sCNTFR for the binding of radiolabelled CNTF to its receptor complex. (A) The SK-N-GP cell line was incubated in the presence of 0.6 nM iodinated CNTF and increasing concentrations of unlabelled CNTF (closed circles), CLC–sCNTFR (closed squares) or IL-2 (open squares). After a 2 h incubation period at 4°C, the bound radioactivity was separated by centrifugation through an oil layer. Tyrosine phosphorylation of gp130, LIFR and STAT3 occurred in SK-N-GP neuroblastoma (gp130+LIFR+CNTFR+) (B) and in HepG-2 hepatoma cell lines (gp130+LIFR+CNTFR–) (C). The SK-N-GP and HepG2 cell lines were incubated for 10 min in the presence of 50 ng/ml LIF, CNTF, CLC–sCNTFR or without cytokine. After cell lysis in 1% Brij 96, LIFR and associated proteins were immunoprecipitated using the AN-E1 anti-LIFR antibody. Tyrosine phosphorylated proteins were analysed by SDS–PAGE followed by western blotting using an anti-phosphotyrosine mAb. After stripping, the membrane was re-stained using an anti-LIFR polyclonal antibody. For determination of tyrosine phosphorylated STAT3, cells were lysed in 1% NP-40. The proteins were then immunoprecipitated using the 4G10 anti-phosphotyrosine mAb, electrophoresed by SDS–PAGE, and western blotted with an anti-STAT3 polyclonal antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.